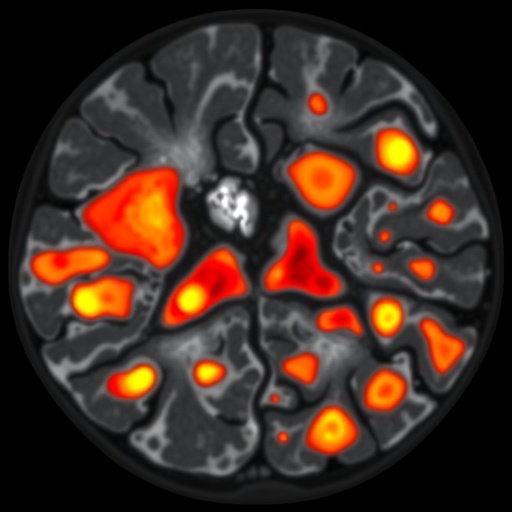
In a groundbreaking study published in Nature Genetics, researchers at The University of Texas MD Anderson Cancer Center have unveiled a complex landscape within diffuse large B-cell lymphoma (DLBCL), revealing seven distinct cellular microenvironments that coexist within these tumors. This innovative investigation provides an unprecedented level of insight into how tumor cells interact with local immune cells, ultimately shaping the treatment responses seen in this aggressive form of non-Hodgkin lymphoma. By illuminating the intricate immune-tumor cross-talk, these findings pave the way for a new era of immune-informed therapies tailored to individual tumor niches.
DLBCL is notorious for its biological variability and clinical heterogeneity, often displaying profound differences in how the cancer cells behave and respond to therapy. Until now, the factors underlying this variability remained poorly understood. Leveraging advanced spatial transcriptomic and proteomic technologies, the MD Anderson team analyzed 78 DLBCL tumor samples, mapping where specific genes and proteins are activated within the tumor tissue microenvironment. Their approach went beyond traditional bulk tumor profiling by preserving the spatial context of immune and malignant cells, thus capturing the dynamic cellular neighborhoods that influence tumor evolution and immune surveillance.
Central to their discovery was the identification of seven distinct microenvironments or “niches,” each characterized by a unique cellular composition and communication network between malignant B cells and various immune cell populations. These niches ranged from immune-depleted zones dominated by tumor cells to highly inflamed regions where immune cells, especially T lymphocytes, infiltrate robustly and show signs of activation. Intriguingly, tumors developing in immune-privileged sites—areas normally shielded from immune attack, such as the central nervous system—exhibited a remarkable paradox: despite their immune-privileged status, these tumors harbored a dense population of T cells deeply interspersed with malignant B cells, alongside gene expression signatures indicative of T cell activation and cytotoxic potential.
This revelation challenges traditional concepts that immune-privileged tumors are completely isolated from immune surveillance. Instead, it suggests that these tumors cultivate active immune microenvironments wherein T cells are poised to recognize and potentially attack tumor cells, although the mechanisms enabling tumor evasion and survival remain to be elucidated. These findings also highlight the importance of spatial context in tumor immunology; understanding where and how immune cells interact with cancer cells within the anatomical landscape is critical to interpreting their functional roles and therapeutic potential.
The study’s meticulous dissection of DLBCL’s cellular architecture underscores why patients with seemingly similar tumors often experience vastly different clinical outcomes. Each microenvironment represents a distinct battleground where tumor cells and immune components engage in a complex dialogue, influencing tumor progression or regression. This heterogeneity likely contributes to the varied therapeutic responses observed with standard chemotherapy and emerging immunotherapies. Consequently, targeting only the malignant B cells without considering the supportive or suppressive immune milieu may fall short of achieving durable remissions.
Furthermore, the characterization of inflammatory niches where T cells are already active reveals promising avenues for therapeutic intervention. According to Linghua Wang, M.D., Ph.D., co-lead author and professor of Genomic Medicine, these “inflammatory pockets” provide clear, tractable targets for therapies designed to harness the patient’s own immune system alongside conventional treatments. Such strategies might include immune checkpoint inhibitors, T cell engagers, or novel immunomodulatory agents that amplify existing anti-tumor immune activity in these niches.
Equally significant is the potential to stratify patients based on their tumor microenvironment profile, allowing clinicians to personalize treatment regimens. Immune-rich tumors might benefit from immunotherapeutic approaches, while immune-poor niches could require microenvironment-modifying agents to recruit or activate immune cells before immunotherapy is effective. This tailored approach could optimize efficacy and minimize unnecessary toxicity by aligning therapy to the tumor’s unique cellular ecosystem.
The research also underscores the value of integrating spatial biology tools into cancer diagnostics. Mapping gene and protein activity within the spatial architecture of tumors provides a multidimensional view of tumor biology, far surpassing the information gleaned from traditional immunohistochemistry or bulk genomic assays. As technologies such as spatial transcriptomics and multiplex imaging mature, they promise to deliver precise immune and tumor cell maps that will guide next-generation precision oncology.
Looking ahead, the MD Anderson team envisions expanding their analysis to larger patient cohorts, incorporating longitudinal sampling to monitor how microenvironments evolve under therapeutic pressure. Understanding the temporal dynamics of immune-tumor interactions will be crucial in optimizing treatment timing and combinations. Moreover, dissecting molecular pathways within distinct immune niches may identify novel targets for drug development, driving a new wave of immunotherapy innovation.
The implications of these findings extend beyond DLBCL, as similar microenvironmental architectures may underlie other hematologic malignancies and solid tumors. By decoding the language of cellular niches, researchers can uncover universal principles of tumor-immune interplay, enabling the design of broadly applicable immunotherapeutic strategies while refining tumor-specific approaches.
In summary, the identification of seven distinct tumor microenvironments in DLBCL represents a major milestone in lymphoma research. This detailed immune landscape mapping not only elucidates the biological basis for tumor heterogeneity and therapeutic resistance but also informs the rational design of immune-engaging therapies tailored to individual patients. As the oncology field moves toward more precise and effective treatments, understanding and targeting the local tumor microenvironment will prove indispensable in transforming outcomes for patients with DLBCL and beyond.
Subject of Research: Diffuse Large B-Cell Lymphoma (DLBCL) tumor microenvironment and immune interactions
Article Title: Distinct Cellular Niches Define Immune Landscapes in Diffuse Large B-Cell Lymphoma
News Publication Date: 2025 (exact date not provided)
Web References: Nature Genetics Article
References: MD Anderson Cancer Center study published in Nature Genetics
Image Credits: Not specified
Keywords: Diffuse Large B-Cell Lymphoma, Tumor Microenvironment, Immune Landscape, T Cell Activation, Spatial Transcriptomics, Immunotherapy, Non-Hodgkin Lymphoma, Cancer Heterogeneity, Immune Privilege, Tumor Immunology
Tags: advanced proteomic technologies in oncologycellular heterogeneity in lymphomadiffuse large B-cell lymphoma treatmentDLBCL biological variabilityimmune-informed cancer therapiesimmune-rich tumor microenvironmentsimmune-tumor interactions in DLBCLMD Anderson Cancer Center research findingspersonalized therapies for lymphomaspatial analysis in cancer studiesspatial transcriptomics in cancer researchtumor microenvironment analysis