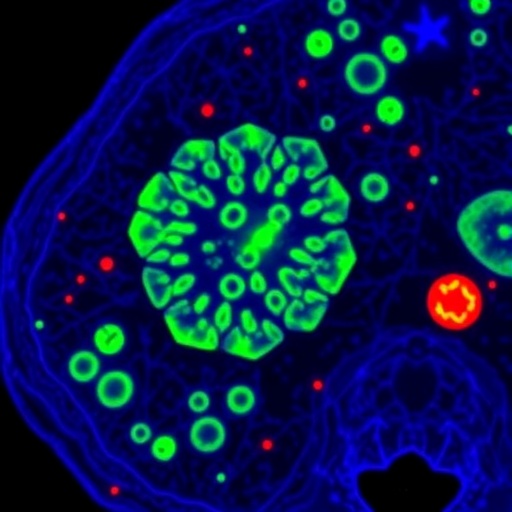
A groundbreaking advancement in dermatological diagnostics has emerged with the development of a novel fluorescent molecular contrast agent known as PARPi-FL. This innovative compound enables the rapid and noninvasive detection of basal cell carcinoma (BCC), the most prevalent form of skin cancer, directly through intact human skin. According to preclinical investigations published in the August 2025 issue of The Journal of Nuclear Medicine, PARPi-FL can reliably identify BCC lesions within five minutes following topical application to excised human tissues, marking a significant leap forward in skin cancer diagnostics.
The conventional diagnostic pathway for basal cell carcinoma involves invasive biopsy procedures, which remain the gold standard for definitive diagnosis. However, biopsies are hampered by several limitations: they are painful, can leave lasting scars, and impose delays due to the time required for histopathological analysis. Such delays can postpone much-needed treatment and may necessitate multiple patient visits. In response to these challenges, researchers have been pursuing noninvasive alternatives equipped with high diagnostic specificity and sensitivity to minimize unnecessary biopsies and expedite clinical decision-making.
PARPi-FL functions as a fluorescent inhibitor targeting the enzyme poly(ADP-ribose) polymerase 1 (PARP1), an enzyme notably overexpressed in numerous cancers, including basal cell carcinoma and melanoma. By fluorescently labeling PARP1, PARPi-FL serves as a molecular beacon, illuminating cancerous cells when observed under fluorescent confocal microscopy. This targeted imaging approach exploits the molecular pathology of skin tumors, allowing clinicians a direct visual assessment of malignancy without the need for tissue extraction.
.adsslot_pAZmRSICUH{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_pAZmRSICUH{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_pAZmRSICUH{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
In a comprehensive study conducted at Memorial Sloan Kettering Cancer Center, the research team meticulously evaluated the optimal parameters for PARPi-FL application, including effective dosage and contact time. Utilizing ex vivo human tissues from diverse sources—such as plastic surgery excisions, Mohs micrographic surgery specimens, and fresh surgical resections—the investigators confirmed that a minimal topical dose of 10 micromolar applied for a duration of two to five minutes provided sufficient dermal penetration and fluorescent signal intensity. The rigorous optimization process ensured a balance between maximal tumor contrast and minimal background staining.
Notably, the fluorescent signal yielded by PARPi-FL in basal cell carcinoma lesions was robust and distinctively higher than in adjacent benign tissues, underscoring its potential for accurate tumor delineation. This contrast specificity is critical to clinical utility, as it suggests that the agent can be harnessed to discriminate malignant from non-malignant skin structures in real time. Such specificity paves the way for the possible reduction of biopsies in cases of ambiguous skin lesions, sparing patients the drawbacks of invasive tissue sampling.
Safety considerations were central to the preclinical evaluation, with toxicology studies demonstrating that topical application of PARPi-FL exhibited no adverse effects on skin integrity or systemic toxicity. This lack of toxicity is particularly promising, as it supports the feasibility of translating the agent into clinical settings without necessitating complex safety procedures or prolonged patient monitoring. The noninvasive nature of this technique positions it as an ideal candidate for point-of-care diagnostics.
Beyond excised human tissue studies, the research incorporated in vivo experiments utilizing tumor-bearing murine models. The topical application method employed gauze pads saturated with PARPi-FL, providing a clinically relevant approach for dye delivery. Real-time imaging was performed with commercially available fluorescent confocal microscopy devices, highlighting the compatibility of PARPi-FL with existing optical imaging platforms. These findings collectively demonstrate the practicality of integrating this molecular imaging agent into hospital and outpatient dermatology clinics.
The implications of this research extend beyond basal cell carcinoma. Given that PARP1 is similarly overexpressed in melanoma cells, there is compelling potential for adapting the PARPi-FL imaging technique to identify and monitor malignant melanoma. This extension would represent a significant advance in the differential diagnosis of pigmented skin lesions, which remain a clinical challenge due to their morphological diversity and overlapping characteristics with benign nevi.
Integration of PARPi-FL into clinical practice could revolutionize dermatological oncology by enabling a “one-stop-shop” solution, where diagnosis and therapeutic decisions can be made rapidly at the bedside. This transformation promises to bridge the gap between diagnosis and treatment, circumventing time-consuming biopsies and histopathology reports. Coupled with emerging nonsurgical treatment modalities for early basal cell carcinoma, such as topical immunotherapy and targeted small molecules, this tool may herald a new era of precision dermatology.
The research team responsible for this development includes multidisciplinary experts from Memorial Sloan Kettering Cancer Center and New York Medical College, reflecting the collaborative nature of molecular imaging advancements. Their collective efforts illuminate pathways for deploying targeted molecular contrast agents to improve cancer diagnostics, underscoring the synergy between optical engineering, pathology, and clinical dermatology.
In essence, the introduction of PARPi-FL marks an exciting convergence of molecular biology and imaging technology. Its rapid diagnostic capability, high specificity, safety profile, and adaptability to clinical imaging systems render it a potent candidate for transforming skin cancer management pathways. Continued development and clinical trials are anticipated to validate these promising results, potentially setting a new standard for noninvasive oncologic diagnostics in dermatology.
Subject of Research: Molecular imaging for noninvasive diagnosis of basal cell carcinoma using a fluorescent PARP1 inhibitor.
Article Title: Open Access Translational Potential of Fluorescent PARP1 Inhibitor as a Molecular Contrast Agent for Diagnosis of Basal Cell Carcinoma.
News Publication Date: August 21, 2025.
Web References:
DOI link to the article: http://dx.doi.org/10.2967/jnumed.124.269428
Journal of Nuclear Medicine website: https://jnm.snmjournals.org/
Image Credits: Image created by Manu Jain and Ashish Dhir, Memorial Sloan Kettering Center, NYC, USA.
Keywords: Molecular imaging, Medical imaging, Skin cancer.
Tags: basal cell carcinoma detectionbiopsy alternatives for skin cancerdermatological advancements in cancerfluorescent imaging methodinnovative cancer detection methodsJournal of Nuclear Medicine findingsnoninvasive skin cancer diagnosticsPARP1 enzyme targeting in cancerPARPi-FL molecular contrast agentpreclinical investigations in dermatologyrapid identification of BCC lesionsskin cancer diagnostic technology