In a groundbreaking study published recently in Nature Metabolism, researchers have unveiled innovative fluorescent dual agonist probes targeting GLP1R and GIPR receptors, illuminating previously elusive cellular landscapes within the pancreas and brain. These probes promise to revolutionize our understanding of incretin hormone action at the cellular level and open new avenues for therapeutic intervention in metabolic diseases, including type 2 diabetes and obesity. By combining cutting-edge molecular design with advanced imaging techniques, this work not only deepens biological insight but also sets a precedent for the development of multifunctional receptor-targeting tools.
The targets of this investigation, glucagon-like peptide-1 receptor (GLP1R) and glucose-dependent insulinotropic polypeptide receptor (GIPR), have long been focal points in metabolic research owing to their integral role in glucose homeostasis. Both receptors mediate the incretin effect, which enhances insulin secretion in response to nutrient intake. Drugs that activate these receptors, either singly or in combination, form the basis of several new diabetes therapeutics, highlighting the translational relevance of wholly understanding their tissue distribution and cellular engagement.
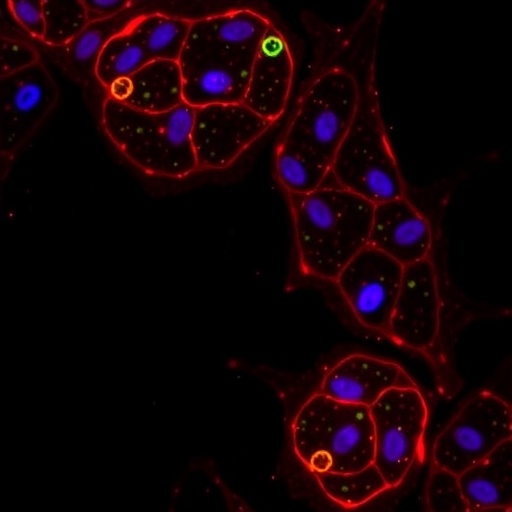
What sets this study apart is its employment of dual agonist probes that are fluorescently labeled, allowing direct visualization of receptor engagement in living tissues. Traditional methods to study receptor activity often relied on indirect readouts or post-mortem analyses, limiting spatial and temporal resolution. The novel probes developed by de Bray et al. overcome these hurdles, offering a direct, sensitive, and dynamic window into receptor localization and function.
.adsslot_XRB6nkioKL{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_XRB6nkioKL{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_XRB6nkioKL{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The technical underpinning of this advance lies in a chemically engineered molecular platform whereby dual receptor agonism is fused to fluorescent moieties without compromising bioactivity. The design challenge was formidable: ensuring that fluorescent tagging did not sterically or electronically hinder the ligand’s affinity and efficacy toward both GLP1R and GIPR. Through meticulous optimization, the study team achieved a balance, producing probes that retain potent agonism while emitting strong fluorescence charge coupled device (CCD)-detectable signals.
Applying these probes in murine pancreatic tissue illuminated complex receptor expression patterns among islet cells. Contrary to the simplistic model of receptor distribution, results revealed heterogenous expression profiles, with GLP1R predominantly marking beta cells and GIPR displaying broader cellular expression. This nuanced landscape suggests that incretin hormones may exert diverse, cell-specific effects previously underappreciated in pancreatic physiology.
Beyond the pancreas, the probes adeptly mapped receptor presence within discrete regions of the brain, areas critically implicated in appetite regulation and energy balance. The fluorescent signals provided compelling visual evidence of receptor colocalization and segregation, offering a cellular framework for understanding central effects of incretins that underlie their influence on feeding behavior and body weight regulation.
Importantly, in vivo imaging demonstrated the probes’ suitability for non-invasive tracking of receptor engagement over time, a landmark achievement that lays the groundwork for longitudinal studies in metabolic disease progression and drug efficacy. Being able to ‘see’ how receptor dynamics shift in response to physiologic or pharmacologic challenges will catalyze precision medicine efforts and biomarker discovery.
The utility of these fluorescent dual agonists extends into pharmacological screening as well, where real-time receptor-ligand binding and downstream signaling cascades can be monitored in living cells with unprecedented clarity. This capability will expedite the identification and refinement of next-generation therapeutics targeting incretin pathways, potentially leading to improved efficacy and reduced side effects.
Moreover, the study highlights subtle differences in ligand-receptor interaction kinetics between pancreatic and neural tissues, hinting at tissue-specific pharmacodynamics that may inform dose and delivery considerations for incretin-based drugs. Understanding these differential mechanisms is crucial for tailoring interventions to maximize therapeutic benefits while minimizing off-target effects.
Beyond their immediate biomedical impact, these probes exemplify a paradigm shift in receptor biology research — from static to dynamic visualization, from one-dimensional to multiplexed receptor interrogation. By integrating fluorescent dual agonism with advanced microscopy, researchers can now dissect complex signaling networks in vivo with spatial and temporal accuracy previously unattainable.
This breakthrough also sets the stage for expanding similar dual-functional fluorescent probes to other receptor systems implicated in chronic diseases, potentially transforming how cellular receptor biology is interrogated across disciplines. The modular nature of the chemical design suggests that such probes could be customized to various receptor pairs, enabling multiplexed imaging strategies.
Crucially, the researchers painstakingly validated probe specificity, ensuring that observed fluorescent signals correspond faithfully to GLP1R and GIPR engagement. This validation involved rigorous controls including receptor knockout models and competitive ligand displacement, safeguarding data integrity and fostering confidence in experimental conclusions.
The implications of these findings resonate beyond fundamental biology and preclinical research; they bear significant translational promise. With incretin-based therapies already in clinical use, enhanced understanding of receptor distribution and dynamics could refine patient stratification, optimize dosing regimens, and mitigate adverse effects, particularly in heterogeneous populations.
Furthermore, the visualization of incretin receptors in the brain provides fresh impetus for exploring their role in neurodegenerative and psychiatric disorders. There is growing interest in incretin signaling as a modulatory axis in neuroinflammation and cognitive function, and these fluorescent probes create new possibilities to study such pathways in vivo.
The study exemplifies the synergy of interdisciplinary collaboration, melding chemical biology, imaging technology, and endocrinology to solve pressing biomedical questions. It stands as a testament to how innovative molecular tools can transform our grasp of complex physiological networks, propelling the field toward more precise and effective interventions.
In summary, de Bray and colleagues have delivered a pioneering technology enabling the real-time, high-resolution visualization of GLP1R and GIPR engagement in living tissues. Their fluorescent dual agonist probes emerge as potent instruments for exploring incretin biology, with vast potential to impact diabetes care, obesity treatment, and brain-related metabolic research. This work not only reveals hidden cellular topographies obscure until now but also charts an exciting course for future receptor-targeted drug development.
As the scientific community absorbs these insights and embraces this new technology, we can anticipate a cascade of discoveries redefining our approach to metabolic regulation and beyond. The confluence of innovative chemistry and biological inquiry embodied by this study exemplifies the future of biomedical research—dynamic, precise, and illuminating at levels once deemed inaccessible.
Article Title:
Fluorescent GLP1R/GIPR dual agonist probes reveal cell targets in the pancreas and brain
Article References:
de Bray, A., Roberts, A.G., Armour, S. et al. Fluorescent GLP1R/GIPR dual agonist probes reveal cell targets in the pancreas and brain.
Nat Metab (2025). https://doi.org/10.1038/s42255-025-01342-6
Image Credits: AI Generated
Tags: advanced molecular designbrain cell mappingcellular visualization methodsfluorescent dual agonist probesGLP1R and GIPR receptorsglucose homeostasis mechanismsincretin hormone actionmetabolic disease researchobesity treatment innovationspancreatic imaging techniquesreceptor-targeting tools developmenttype 2 diabetes therapeutics