A groundbreaking multicenter study has unveiled a cutting-edge radiomics model utilizing contrast-enhanced ultrasound (CEUS) imaging to accurately predict axillary lymph node metastasis (ALNM) and patient prognosis in breast cancer. This innovative approach heralds a significant leap forward in personalized cancer diagnostics, offering clinicians a powerful tool to assess tumor progression and tailor therapy with unprecedented precision. By integrating multimodal ultrasound imaging data and advanced machine learning algorithms, researchers have crafted a predictive framework surpassing traditional imaging techniques in both accuracy and prognostic utility.
The research team collected comprehensive data from 682 breast cancer patients diagnosed between 2014 and 2022 across four major hospitals in China. They compiled preoperative grayscale ultrasound (US), color Doppler flow imaging (CDFI), and contrast-enhanced ultrasound (CEUS) scans alongside critical clinical information. This rich dataset laid the groundwork for developing a multifaceted radiomics model aimed at detecting ALNM—a vital prognostic indicator that directly influences treatment decisions and survival outcomes in breast cancer patients.
Axillary lymph node involvement remains a pivotal determinant in breast cancer staging and therapy planning. Conventionally, detecting metastasis relies on invasive procedures like sentinel lymph node biopsy, often fraught with complications and patient burden. The advent of noninvasive radiomics models that extract quantitative imaging features from ultrasound modalities introduces a paradigm shift—enabling precise preoperative prediction and potentially reducing unnecessary surgical interventions.
.adsslot_FcuLMsoCtW{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_FcuLMsoCtW{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_FcuLMsoCtW{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Central to the study’s methodology was the application of Least Absolute Shrinkage and Selection Operator (LASSO) regression to distill a vast array of radiomic features embedded within ultrasound images. This statistical technique refined feature selection by eliminating redundancies and identifying those most predictive of ALNM. Subsequently, eight distinct machine learning algorithms were utilized to construct radiomics models drawing on US, CDFI, and CEUS datasets individually and in combination, providing a robust framework to evaluate predictive performance.
The results demonstrated that the combined US + CDFI + CEUS model significantly outperformed ultrasound-only assessments in predicting the presence and extent of metastatic axillary lymph nodes. Specifically, the integrated radiomics model achieved Areas Under the Curve (AUCs) of 0.88 in the training set and maintained strong predictive accuracy in both internal and external validation cohorts, with AUCs exceeding 0.75. These statistics underscore the reliability and generalizability of the approach across diverse clinical environments.
Beyond binary classification of lymph node status (N0 versus N+), the model adeptly distinguished between varying metastatic burdens—a crucial clinical nuance. It achieved high AUCs when differentiating patients with 1–2 positive nodes from those with three or more, highlighting its ability to stratify patients based on metastatic extent. This granularity facilitates more tailored therapeutic regimens, optimizing outcomes while minimizing overtreatment.
Importantly, the study also explored the prognostic implications of integrating radiomics with systemic immunoinflammatory markers, including platelet count and neutrophil-to-lymphocyte ratio (NLR). Such markers reflect tumor-host interactions and the underlying inflammatory milieu, which are increasingly recognized as key determinants of cancer progression. By combining the radiomic signature (Radscore) with these clinical biomarkers, the researchers developed a composite model predicting disease-free survival (DFS) with notable accuracy.
This composite clinical-radiomics model exhibited C-indices ranging from 0.73 to 0.80 across different cohorts, outperforming models based solely on clinical data. In external validations, it demonstrated superior AUCs for predicting 2-, 3-, and 5-year DFS outcomes compared to clinical models alone, with improvements reaching statistical significance. Such advances enable oncologists not only to forecast metastatic spread but also to anticipate patient prognosis, facilitating more informed counseling and individualized surveillance strategies.
The utility of the model was further bolstered by rigorous calibration and decision curve analyses, which confirmed its clinical applicability and net benefit. Good agreement between observed and predicted outcomes indicated that the model’s predictions closely mirrored real-world patient trajectories. Decision curve analysis demonstrated tangible clinical benefits over existing tools, suggesting that adopting this radiomics-based approach could enhance decision-making in breast cancer management.
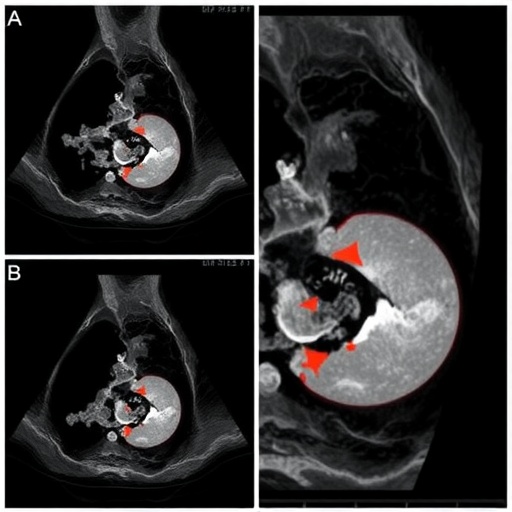
At the core of the innovation lies the incorporation of contrast-enhanced ultrasound, which augments traditional imaging by highlighting microvascular perfusion characteristics within suspicious lesions and nodes. CEUS provides dynamic functional information beyond morphological assessment, enabling extraction of texture and intensity-based radiomic features deeply linked to tumor biology and metastatic potential. This multimodal imaging strategy thus enriches data granularity and model robustness.
The study’s multicenter design and large patient cohort contribute to the robustness and external validity of findings. Data partitioning ensured rigorous training, internal validation, and external validation phases, mitigating overfitting risks—a common pitfall in radiomics research. The diverse population sampled from geographically and institutionally distinct centers strengthens the generalizability of conclusions, paving the way for broader clinical adoption.
Nonetheless, the authors acknowledge that further prospective studies are warranted to validate the model’s performance in routine clinical workflows and to assess integration feasibility with existing diagnostic protocols. Future research may also explore combining ultrasound radiomics with genomic or molecular biomarkers, fostering an integrative oncology paradigm blending imaging phenotypes with biological insights.
Moreover, the technology holds promise for guiding neoadjuvant therapy decisions, surgical planning, and personalized follow-up schedules. By accurately delineating lymph node status preoperatively, the model may reduce the need for invasive biopsies, lowering patient morbidity and healthcare costs. Predicting prognosis through combined imaging and inflammatory markers offers a noninvasive avenue to identify high-risk patients who may benefit from intensified treatments.
This advancement epitomizes the transformative potential of artificial intelligence and machine learning in oncology, leveraging routinely acquired imaging data to extract clinically actionable insights. As ultrasound is widely available, radiation-free, and cost-effective, deploying enhanced radiomics models such as this could democratize access to precision diagnostics worldwide, especially in resource-limited settings.
The study epitomizes a confluence of medical imaging, computational analysis, and clinical oncology, showcasing how interdisciplinary efforts can unlock novel diagnostic and prognostic capabilities. By converting complex imaging data into predictive models that inform real-world treatment pathways, research like this accelerates the transition from traditional medicine to precision oncology.
In summary, the novel multimodal ultrasound radiomics model combining grayscale US, CDFI, and CEUS represents a formidable tool in the battle against breast cancer. It not only elevates the accuracy of axillary lymph node metastasis detection but also synergizes with immunoinflammatory biomarkers to enhance prognosis prediction. Such integrative models promise to optimize patient stratification, personalize therapy, and ultimately improve survival and quality of life for countless breast cancer patients worldwide.
As this technology matures and is incorporated into clinical practice, it may redefine breast cancer care pathways, reducing diagnostic uncertainties and empowering clinicians with nuanced risk assessments. The synergistic blend of advanced imaging, machine learning, and biological markers portends a new era of precision oncology where treatment decisions are increasingly guided by data-driven insights.
This landmark study stands as a testament to the potential of radiomics combined with immunology to revolutionize cancer prognostication. It paves the way for future endeavors harnessing artificial intelligence to unravel complex disease patterns from noninvasive imaging, transforming clinical oncology from reactive to proactive, and setting new standards in patient-centered care.
Subject of Research:
Prediction of axillary lymph node metastasis and prognosis in breast cancer using multimodal ultrasound radiomics combined with immunoinflammatory markers.
Article Title:
Contrast-enhanced ultrasound radiomics model for predicting axillary lymph node metastasis and prognosis in breast cancer: a multicenter study.
Article References:
Li, S.Y., Li, Y.M., Fang, Y.Q. et al. Contrast-enhanced ultrasound radiomics model for predicting axillary lymph node metastasis and prognosis in breast cancer: a multicenter study. BMC Cancer 25, 1315 (2025). https://doi.org/10.1186/s12885-025-14632-9
Image Credits: Scienmag.com
DOI:
https://doi.org/10.1186/s12885-025-14632-9
Tags: axillary lymph node metastasisbreast cancer metastasis predictionbreast cancer stagingcontrast-enhanced ultrasoundmachine learning in oncologymultimodal ultrasound imagingnoninvasive imaging techniquespersonalized cancer diagnosticspredictive framework for cancer therapyprognostic utility in breast cancersentinel lymph node biopsy alternativesultrasound radiomics