In a groundbreaking study published recently in Nature Communications, researchers from the Rheinland-Pfälzische Technische Universität Kaiserslautern-Landau (RPTU) have unveiled a novel mechanistic link between aneuploidy-induced proteotoxic stress and mitochondrial dysfunction. Their work elucidates how the presence of extra chromosomes in cells disrupts mitochondrial homeostasis by promoting the aggregation of mitochondrial precursor proteins via the sequestosome 1 (SQSTM1/p62) pathway. This discovery not only advances our molecular understanding of chromosomal abnormalities common in cancer and genetic syndromes but also opens potential avenues for targeted therapies to address mitochondrial dysfunction and proteostasis imbalance in aneuploid diseases.
Each human somatic cell typically harbors 23 pairs of chromosomes, ensuring the accurate distribution of genetic material during cell division. Aneuploidy refers to the condition where cells possess abnormal numbers of chromosomes, either as an addition or loss of entire chromosomes. This chromosomal imbalance is notorious in oncogenesis and certain genetic disorders such as Down syndrome. The RPTU research consortium spearheaded by Professor Zuzana Storchová has shed light on how even subtle numerical deviations in chromosomes initiate cascades of biochemical disturbances that severely impact cellular organelles, particularly mitochondria.
Previous knowledge has established that abnormal chromosome numbers induce an imbalance in protein synthesis, overwhelming cellular quality control systems that maintain proteome equilibrium. However, the specific downstream consequences of these proteostatic disruptions—especially on mitochondrial function—have remained elusive. Using sophisticated genetic engineering, the team generated colorectal cancer cell lines with one or two additional chromosomes by modifying the near-diploid HCT116 background, thereby creating controlled models of aneuploidy to dissect cellular consequences at high resolution.
.adsslot_Ka4L1xY3yc{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_Ka4L1xY3yc{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_Ka4L1xY3yc{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
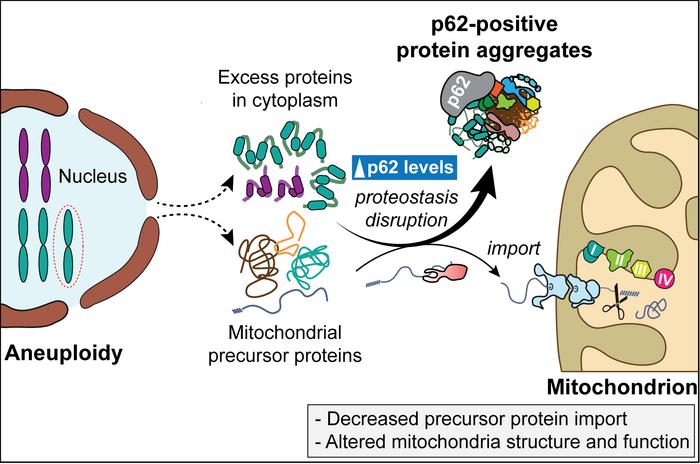
Intriguingly, these aneuploid cell lines accumulated cytoplasmic protein aggregates lacking delimiting membranes. Central to these aggregates was SQSTM1, also known as p62, a multifunctional autophagy receptor known to bind ubiquitinated proteins and damaged organelles for degradation. The research demonstrated that the quantity of p62-containing aggregates positively correlated with the size of the extra chromosome, suggesting that chromosome dosage directly influences proteostasis stress levels. This finding highlights p62’s pivotal role in managing the cellular burden induced by superfluous protein synthesis due to aneuploidy.
Delving deeper, the study uncovered that mitochondrial precursor proteins—normally synthesized in the cytosol and imported into mitochondria for proper function—were sequestered within p62-positive aggregates. This sequestration effectively ‘confiscated’ these precursors, preventing their timely translocation into mitochondria. As a consequence, mitochondrial architecture and bioenergetics deteriorated, signifying a profound disruption of mitochondrial homeostasis triggered by chromosomal imbalances. This molecular crosstalk between nuclear genome instability and mitochondrial dysfunction marks a critical insight into aneuploidy pathology.
The researchers emphasize that their engineered cell lines mimic physiologically relevant aspects of human pathologies characterized by unbalanced genomic content, including cancer and trisomy syndromes. This experimental platform thus represents an invaluable tool for investigating how proteome imbalances translate into organelle dysfunction. The implications extend beyond basic biology, as the intersection of proteotoxic stress and impaired mitochondrial metabolism may underlie the remarkable adaptability and drug resistance observed in aneuploid cancer cells.
Interestingly, despite the inherent stress of chromosome number abnormalities, cancer cells often sustain viability and proliferative capacity by modulating mitochondrial functions. The team hypothesizes that alterations in mitochondrial metabolism may serve as an adaptive mechanism to withstand the detrimental consequences of proteotoxic stress, thereby conferring survival advantages under chemotherapeutic pressures. This paradigm challenges the traditional view of cancer cells as merely victims of proteome imbalance, instead highlighting their dynamic metabolic plasticity.
Professor Storchová articulates the broader significance of these findings by stating that they reveal a previously unappreciated linkage between genomic aberrations, protein aggregation stress, and mitochondrial function. Such insights underscore the intricate interdependence between cellular compartments and quality control systems in preserving cell viability amidst genetic disturbances. By delineating the molecular pathways involved, this research paves the way for exploring targeted interventions aimed at restoring mitochondrial import and alleviating proteotoxic aggregation in aneuploid conditions.
In addition to providing mechanistic clarity, the study emphasizes translational potential. The aggregation of mitochondrial precursor proteins via the SQSTM1/p62 axis could represent a druggable vulnerability in cancers harboring chromosomal abnormalities. Therapeutic strategies aimed at modulating p62-mediated pathways or enhancing mitochondrial protein import efficiency may sensitize tumor cells to existing treatments and overcome intrinsic drug resistance. As such, the work offers a promising framework for improving oncological outcomes by exploiting the mitochondrial adaptations of aneuploid cells.
This research was conducted as part of the graduate school program STRESSistance at RPTU, funded by the German Research Foundation, and benefited from collaborations with research groups specializing in molecular genetics, cell biology, and systems biology of neurodegenerative diseases. Cross-institutional cooperation facilitated a comprehensive approach, combining genetic engineering, biochemical assays, and advanced imaging techniques to unravel the cellular consequences of aneuploidy with unprecedented detail.
First author and postdoctoral researcher Prince Saforo Amponsah played a critical role in leading the project and conceptualizing the mitochondrial sequestration mechanisms. Supported by prestigious fellowships and grants, Amponsah’s work stands at the intersection of molecular genetics and cellular bioenergetics, spearheading efforts to understand how genomic instability impacts organelle function. His contributions highlight the importance of interdisciplinary research in tackling complex biological phenomena relevant to cancer and genetic disorders.
In conclusion, the pioneering study challenges existing notions about the biological repercussions of aneuploidy by linking protein homeostasis disturbances to mitochondrial dysfunction via p62-dependent protein aggregation. This nexus articulates a molecular pathway that may contribute significantly to disease progression and therapy resistance in cancers and other aneuploid conditions. Future research building upon these insights holds promise for innovative therapeutic strategies targeting mitochondrial adaptations in aberrant genomic contexts.
Subject of Research: Cells
Article Title: Aneuploidy-induced proteostasis disruption impairs mitochondrial functions and mediates aggregation of mitochondrial precursor proteins through SQSTM1/p62
News Publication Date: 17-Jun-2025
Web References: DOI: 10.1038/s41467-025-60857-4
Image Credits: Prince Saforo Amponsah
Keywords: Aneuploidy, Proteostasis, Mitochondrial Dysfunction, SQSTM1, p62, Protein Aggregation, Mitochondrial Precursor Proteins, Cancer, Chromosomal Imbalance, Proteotoxic Stress, Mitochondrial Import, Cellular Metabolism
Tags: abnormal chromosome numbersadvances in molecular biology researchaneuploidy and mitochondrial dysfunctionchromosomal abnormalities and genetic syndromesimpacts of chromosomal imbalance on cell functionimplications for cancer treatment strategiesmechanisms of proteostasis imbalancemitochondrial homeostasis and cellular healthproteotoxic stress in cancerrole of sequestosome 1 in cellsRPTU study on chromosomal disorderstargeted therapies for aneuploid diseases