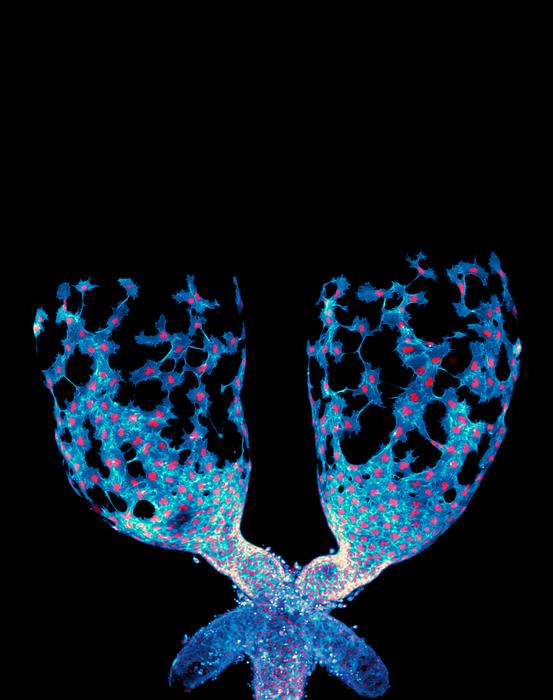
A groundbreaking study conducted by researchers at the University of North Carolina at Chapel Hill has reshaped our understanding of how organs are sculpted during development. Traditionally, organ formation has been attributed to relatively static epithelial cells that build tissue architecture like bricks in a wall. However, this new research highlights the remarkable mobility and coordinated behavior of mesenchymal cells, especially muscle precursor cells, which actively migrate and mold developing organs. Using Drosophila melanogaster, commonly known as fruit flies, as a model organism, the scientists have revealed unprecedented real-time dynamics of organ shaping, specifically focusing on the testis—a structure that transforms from an oval shape into a complex spiral during development.
The researchers utilized advanced live-imaging techniques to visualize the intricate process by which muscle precursor cells crawl over and eventually constrict the testicular tissue. This dynamic migration contrasts sharply with the long-held view of cells as largely passive building blocks. Instead, these mesenchymal cells exhibit collective migration, physically maneuvering and signaling to one another to sculpt the organ in a highly orchestrated manner. The ability to capture these movements live marked a significant leap beyond previous studies, which mostly relied on static tissue snapshots, thus missing the full choreography of cellular interactions during organogenesis.
A particularly surprising aspect of the study is the discovery that the signaling pathways driving this collective migration are ones previously thought exclusive to neural development. Specifically, the Plexin/Semaphorin signaling axis, well known for its role in guiding neuronal wiring, also regulates the balance between epithelial and mesenchymal states in this context. This finding hints at a fascinating evolutionary conservation of cellular communication mechanisms shared across vastly different tissues. The study proposes that molecular systems underpinning brain development might be repurposed in other organs to facilitate complex morphogenetic events.
.adsslot_ENJszZO7xj{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_ENJszZO7xj{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_ENJszZO7xj{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Muscle precursor cells engage in a highly coordinated “crawl” over the testis surface, driven by the tight regulation of cell-cell communication through Plexin/Semaphorin antagonism. This signaling system ensures the appropriate epithelial-mesenchymal balance is maintained, which is crucial for the cells to collectively migrate and sculpt the organ correctly. Disruption of this system, the researchers observed, impairs the shaping process, highlighting the signaling pathway’s critical regulatory role. This research thus places Plexin/Semaphorin signaling at the heart of collective cell migration during organ development, expanding its known biological significance.
The study’s implications extend beyond developmental biology. Mesenchymal cells not only play central roles in normal organ formation but are also implicated in pathological states such as cancer. Cancer cells often acquire mesenchymal characteristics, enabling invasive migration during metastasis. Understanding the molecular machinery regulating mesenchymal cell coordination in normal development could open new avenues to decipher how tumors spread through similar mechanisms. This link underscores the potential translational impact of deciphering mesenchymal dynamics in health and disease.
Live-imaging technology was indispensable for these findings. Observing cells in motion rather than through fixed images revealed dynamic behaviors that were previously impossible to understand. Dr. Maik Bischoff emphasized that inferring developmental processes from static images is akin to learning basketball rules from a few pictures—you miss the fluidity and interaction essential to the game. This approach heralds a methodological shift in developmental biology, emphasizing temporal resolution and real-time cellular interactions.
Furthermore, the study contributes to the emerging view that organ morphogenesis is not solely a product of intrinsic cellular properties but also of intercellular signaling networks coordinating multicellular behavior. This collective migration is akin to an elaborate dance where individual cells communicate and respond to their neighbors to shape functional tissue architecture. Such complexity challenges simplified models of organogenesis and sets the stage for future research to explore how these signaling pathways integrate with genetic and mechanical factors.
A fascinating nuance uncovered by the UNC team involves the way migrating muscle precursor cells transition their behavior over time. Initially highly motile, these cells progressively tighten around the testis, orchestrating a structural transformation from a simple oval to an intricate spiral. This gradual constriction likely involves changes in cytoskeletal tension and adhesion properties, driven by the interplay between Plexin/Semaphorin signaling and downstream effectors. Such findings may inspire novel mechanical models of tissue morphogenesis integrating molecular and biophysical data.
The interdisciplinary nature of this work stands out. Combining expertise in cell biology, developmental biology, live-cell imaging, and molecular signaling, the team achieved a detailed portrayal of a previously elusive phenomenon. Postdoctoral researcher Dr. Maik Bischoff led the imaging and experimental analyses, while senior author Dr. Mark Peifer provided crucial insights into the broader biological and medical relevance. Their collaboration encapsulates how contemporary biological questions demand multifaceted approaches.
By shedding light on previously overlooked mesenchymal cells, the study challenges conventional models that have marginalized these cells as merely supporting players. Instead, mesenchymal cells emerge as powerful architects of tissue form and function, dynamically navigating and remodeling the microenvironment in response to intrinsic signals. This paradigm shift is likely to stimulate renewed interest in mesenchymal biology, with far-reaching consequences for developmental biology, regenerative medicine, and oncology.
The UNC researchers also spotlight the value of model organisms like fruit flies for unraveling conserved biological principles. Despite vast evolutionary distances, Drosophila continues to reveal fundamental cellular behaviors and signaling pathways with direct relevance to human biology. This underscores fruit flies’ enduring role as a cost-effective platform for high-resolution live imaging and genetic manipulation, facilitating discoveries that resonate across species.
As this study demonstrates, the intricate dance of cells during organ formation is a marvel of coordinated signaling and migration. Such insights, powered by technological advances and fresh conceptual frameworks, promise to enrich our comprehension of organogenesis and pave the way for innovative strategies to address developmental disorders and cancer progression. The unfolding story of mesenchymal cell migration is only beginning, but its implications reach far beyond the testis, heralding a new era in understanding biological form and function.
Subject of Research: Cells
Article Title: Plexin/Semaphorin Antagonism Orchestrates Collective Cell Migration and Organ Sculpting by Regulating Epithelial-Mesenchymal Balance.
News Publication Date: 18-Jun-2025
Web References: https://www.science.org/doi/10.1126/sciadv.adu3741
References: 10.1126/sciadv.adu3741
Image Credits: Dr. Maik Bischoff
Keywords: Cells, Cancer cells, Developmental disabilities, Tumor cells
Tags: advanced research methodologiescancer spread insightscellular signaling in organ formationDrosophila melanogaster modeldynamic organ sculptingepithelial versus mesenchymal cellslive-imaging techniques in researchmesenchymal cell migrationmuscle precursor cells behaviororgan development dynamicstestis organ shapingUNC Chapel Hill study