In a groundbreaking study poised to reshape the landscape of cancer immunotherapy, researchers at the VIB-KU Leuven Center for Cancer Biology have unveiled a paradoxical role of ‘natural killer’ (NK) cells in melanoma patients resistant to immune checkpoint blockade (ICB) therapies. Traditionally celebrated as potent cytotoxic agents targeting tumor cells, these NK cells may, under certain circumstances, hinder the immune system’s assault on malignancies. Published in the journal Cancer Discovery, this research elucidates how NK cells act as gatekeepers in the tumor microenvironment, obstructing the infiltration of the immune system’s frontline soldiers, the CD8 T cells, thereby contributing to therapeutic resistance.
Melanoma remains one of the deadliest skin cancers globally, with over 330,000 new cases diagnosed annually and approximately 60,000 deaths attributed to this aggressive malignancy each year. While early detection offers a high curative potential, advanced stage melanomas frequently develop resistance mechanisms that severely limit the effectiveness of existing treatments. Immune checkpoint blockade therapies, which bolster the immune system’s natural ability to recognize and destroy cancer cells, have transformed oncological care. Despite these advances, roughly 50% of patients with advanced melanoma do not respond to ICB, underscoring an urgent need to decipher the underlying biological mechanisms that confer resistance.
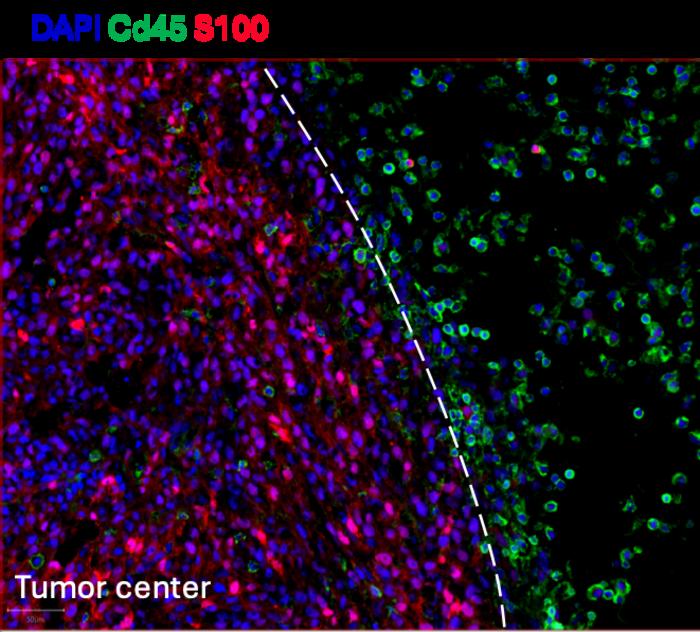
The team led by Professor Jean-Christophe Marine tackled this question by leveraging cutting-edge spatial omics technologies to analyze tumor biopsies from melanoma patients collected before and shortly after the initiation of ICB therapy. These technologies enabled the precise mapping of cellular populations within the tumor microenvironment — information critical for understanding how immune cells interact with malignant cells. The results revealed a surprising and counterintuitive phenomenon: in patients unresponsive to ICB, there was a pronounced increase in cytotoxic NK cells; paradoxically, these immune cells were restricted to the tumor periphery, forming a physical barrier that excluded the infiltration of CD8 T cells, the key effectors responsible for directly killing cancer cells.
.adsslot_xGn0LKFsNY{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_xGn0LKFsNY{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_xGn0LKFsNY{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
In contrast, tumors from patients who showed a positive response to ICB therapy exhibited a markedly different immune cell landscape. NK cells in these responders were found to successfully penetrate the tumor core in conjunction with CD8 T cells. This immune accessibility appeared to correlate directly with tumor clearance, highlighting the critical importance of immune cell spatial distribution in therapeutic outcome. This discovery challenges longstanding dogmas about the universally beneficial roles of NK cells in cancer immunity and suggests that their context-dependent behavior can profoundly influence treatment efficacy.
Dr. Joanna Pozniak, first author of the study, articulated the scientific community’s surprise: “We were astonished to find that NK cells, widely considered cancer fighters, can, under specific conditions, actually prevent T cells from executing their tumoricidal functions. This insight compels a reevaluation of the tumor immune landscape and suggests potential new targets to overcome resistance in patients with limited therapeutic options.”
Seeking to experimentally dissect the role of NK cells in enforcing this immune exclusion, the researchers developed a sophisticated murine melanoma model that mimicked immune-excluded tumors seen in resistant human patients. In this model, when NK cells were pharmacologically depleted, a remarkable shift occurred: CD8 T cells were liberated from their confinement at the tumor periphery, infiltrating the tumor core robustly. This infiltration significantly improved tumor clearance when combined with ICB therapy, confirming that NK cells were indeed acting as a physical and functional barrier to T cell-mediated antitumor immunity.
The mechanistic investigation further revealed that NK cells employ the chemokine receptor CX3CR1 as a molecular “key” to mediate their recruitment and spatial positioning around the tumor. By pharmacologically blocking CX3CR1 signaling, the immune blockade imposed by NK cells was disrupted, allowing CD8 T cells access to the tumor interior and restoring responsiveness to immunotherapy. This finding positions CX3CR1 as a promising therapeutic target that could sensitize resistant tumors and broaden the patient population benefiting from ICB.
Jean-Christophe Marine emphasized the clinical potential of these insights: “Our data suggest that NK cells can act as gatekeepers for T cells, a role previously unappreciated in cancer immunity. Disrupting the CX3CR1-mediated NK cell recruitment pathway may open new therapeutic avenues, enhancing the efficacy of ICB treatments in melanoma patients who currently lack effective options.”
The study was made possible through the VIB Grand Challenges Program’s Pointillism project, which harnesses single-cell multi-omics and spatial profiling to generate unparalleled resolution of tumor ecosystems. In its initial phase, Pointillism identified key biomarkers predictive of responses to checkpoint blockade in both melanoma and breast cancer. These findings laid the groundwork for Pointillism 2.0, which aims to validate and integrate biomarker panels into minimally invasive blood tests, enabling rapid and precise prediction of patient responses to ICB therapies.
Looking ahead, the research team hopes to translate these preclinical findings into clinical interventions that can disrupt the exclusionary NK cell barrier and improve prognosis for melanoma patients. Such advances would mark a significant leap in personalized cancer immunotherapy, addressing a long-standing challenge of therapeutic resistance. As Prof. Marine concluded, “Despite the remarkable progress in cancer treatment over the last decades, many patients remain refractory to current approaches. Our work opens a promising path toward unlocking immunotherapy for a wider cohort, bringing us closer to the goal of overcoming cancer’s formidable defenses.”
This study exemplifies the critical importance of high-resolution spatial mapping and functional interrogation of the tumor microenvironment, illustrating how nuanced cell-cell interactions dictate therapeutic outcomes. By reimagining the role of cytotoxic NK cells from tumor-killing allies to potential immune suppressors, the findings urge the oncology field to refine existing immunotherapy paradigms and highlight new molecular targets to advance cancer care.
Subject of Research: Resistance mechanisms in melanoma to immune checkpoint blockade therapy mediated by natural killer (NK) cells in the tumor microenvironment.
Article Title: Cytotoxic NK cells impede response to checkpoint immunotherapy in melanoma with an immune-excluded phenotype
News Publication Date: 18 June 2025
Web References: DOI link
Image Credits: VIB
Keywords: Melanoma, Immune cells, Natural killer cells, Immune checkpoint blockade, Tumor microenvironment, CD8 T cells, Immunotherapy resistance, CX3CR1, Spatial omics, Cancer immunology
Tags: biological mechanisms of therapeutic resistanceCD8 T cells and cancer treatmentgroundbreaking cancer studiesimmune checkpoint blockade challengesimmune system and cancermelanoma immunotherapy resistancemelanoma patient treatment outcomesnatural killer cells in cancer therapyoncology research advancementsparadoxical roles of immune cells in cancertumor microenvironment and NK cellsVIB-KU Leuven cancer research