In a groundbreaking study that challenges long-held principles of evolutionary biology, Chinese scientists have provided compelling molecular evidence supporting the concept of Lamarckian inheritance, a theory proposing that acquired traits can be passed down through generations. This pioneering research, conducted at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, illuminates how rice plants adapt to cold environments through epigenetic modifications, specifically DNA methylation changes, thereby facilitating their survival and expansion into colder, high-latitude regions.
Published on May 22, 2025, in the prestigious journal Cell, the study focuses on the intricate genetic and epigenetic mechanisms underlying rice’s remarkable ability to acquire and inherit cold tolerance. Led by Professor CAO Xiaofeng, the team developed an innovative multigenerational cold-stress experimental system targeting the sensitive meiotic phase of rice development. Through applying directional selection over three consecutive generations, they successfully isolated rice lines exhibiting dominant inheritance patterns of enhanced cold tolerance, traits that persisted robustly for at least five generations even after the removal of cold stress conditions.
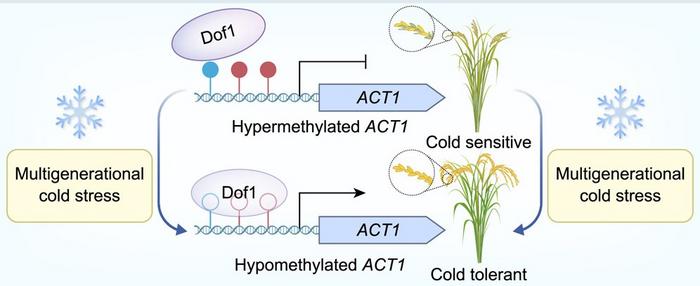
Central to this inheritance pattern is the epigenetic modification of an important gene promoter—the promoter region of the ACT1 gene, which encodes an arabinogalactan protein critically involved in cold stress response. Multi-omics profiling revealed pronounced hypomethylation in the ACT1 promoter, a change that relieves transcriptional repression and amplifies gene expression. Using state-of-the-art DNA methylation editing tools, researchers precisely manipulated the methylation status of the ACT1 promoter, confirming the direct causal relationship between promoter hypomethylation and increased cold tolerance. This precise epigenetic regulation underscores the dynamic interplay between environmental stress and heritable gene expression control.
Delving deeper into molecular mechanisms, the investigators uncovered that exposure to cold stress significantly represses the expression of MET1b, a DNA methyltransferase responsible for maintaining methylation patterns during DNA replication. The downregulation of MET1b disrupts the maintenance of methylation marks at the ACT1 promoter, engendering a hypomethylated epiallele that is inherited by subsequent generations. Importantly, the hypomethylated ACT1 promoter features a binding site for the transcription factor Dof1, whose DNA binding affinity is inversely correlated with methylation status. Functional knockout of Dof1 markedly diminished cold tolerance, establishing a regulatory axis whereby cold stress triggers MET1b repression, leading to promoter hypomethylation, augmented Dof1 binding, and consequent activation of ACT1 expression, culminating in enhanced cold resilience.
Natural variation analysis across 131 rice landraces from diverse Chinese agro-ecological zones further substantiated this epigenetic mechanism’s evolutionary significance. While ACT1 gene coding sequences remained highly conserved among the populations, the DNA methylation status of its promoter exhibited striking geographic polymorphisms aligned with cold tolerance phenotypes. Varieties from the warmer southern and central regions predominantly exhibited hypermethylated, repressed ACT1 promoters, whereas those adapted to the frigid northeastern locales demonstrated a pronounced enrichment of hypomethylated, more transcriptionally active ACT1 alleles. This “south-high, north-low” methylation gradient reflects natural selection favoring epigenetic variants that confer adaptive cold resilience during rice domestication and migration.
Remarkably, the study reveals that this epigenetically encoded cold tolerance operates outside the classical Mendelian framework reliant on DNA sequence mutations, embracing instead a model of non-genomic inheritance. This insight revitalizes the Lamarckian paradigm—once dismissed in mainstream evolutionary discourse—and suggests that epigenetic inheritance mechanisms can facilitate rapid phenotypic adaptation to environmental pressures. The findings highlight the possibility of environmentally induced epigenetic changes being stably transmitted across generations, contributing to evolutionary fitness.
Reviewers commenting on the publication emphasized its paradigm-shifting implications, noting that it expands our understanding of adaptive processes and introduces molecular mechanisms that transcend traditional Darwinian genetic inheritance. By elucidating the causal epigenetic modifications and their direct links to phenotypic adaptations, the study opens exciting avenues to revisit evolutionary theory with a broadened perspective incorporating epigenetics.
Beyond theoretical ramifications, this research carries significant applied potential. The authors propose a novel breeding strategy integrating stress-induced epigenetic variation with precision epigenome editing to accelerate the development of crops resilient to environmental stresses. This “stress domestication → epigenetic variant identification → precision editing” pipeline could revolutionize agriculture, empowering breeders to rapidly tailor phenotypic traits without altering the underlying genome, an approach especially crucial in the face of accelerating climate change.
The evidence supporting heritable epigenetic adaptation in rice provides a compelling template for exploring similar mechanisms in other crops and organisms, potentially transforming strategies for crop improvement globally. By harnessing the epigenome’s plasticity, breeders may overcome limitations imposed by slower, DNA sequence–based mutation rates, thus securing food production amid increasingly volatile climates.
In conclusion, this landmark study from Professor CAO Xiaofeng’s team not only validates the molecular basis of Lamarckian inheritance but also reshapes the conceptual landscape of evolutionary biology and crop science. The demonstration of stable, environmentally induced epigenetic modifications as vehicles for adaptation underscores a sophisticated layer of regulation beyond classical genetics and heralds a new era of precision agriculture that judiciously leverages epigenetic information to cultivate resilient, high-performing crop varieties for future food security.
Subject of Research:
Epigenetic inheritance and adaptive cold tolerance in rice mediated by DNA methylation
Article Title:
Inheritance of acquired adaptive cold tolerance in rice through DNA methylation
News Publication Date:
22-May-2025
Web References:
https://doi.org/10.1016/j.cell.2025.04.036
Image Credits:
IGDB (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences)
Keywords:
Plant genetics, Plant evolution, Plant genes, Plant genomes, Gene expression, Genetic engineering, RNA methylation
Tags: ACT1 gene promoter modificationscold tolerance in agricultureDNA methylation in riceepigenetic mechanisms in rice cold adaptationevolutionary biology and epigeneticsgenetic adaptation to cold stressinnovative research in plant geneticsLamarckian inheritance in plantsmolecular evidence of trait inheritancemultigenerational cold-stress experimentsresilience of rice in high-latitude regionsrice breeding for cold tolerance