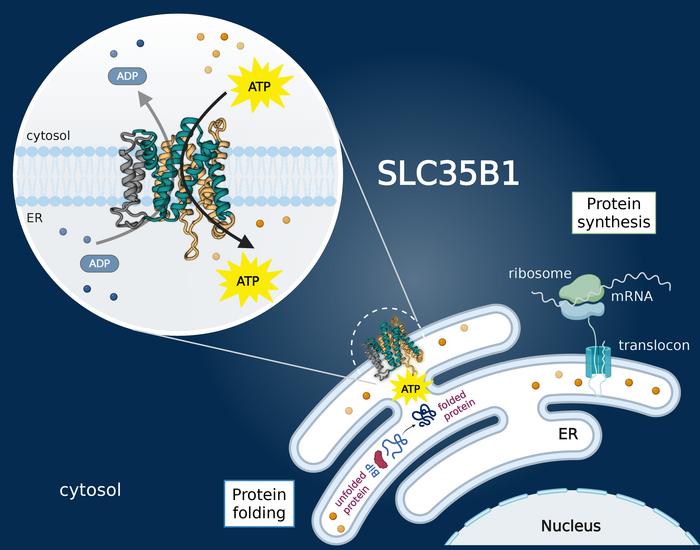
A groundbreaking discovery by a team of biochemists has resolved a fundamental question that has persisted within cell biology for decades: how exactly does adenosine triphosphate (ATP), the cell’s primary energy currency, gain entry into the endoplasmic reticulum (ER)? This enigmatic process has far-reaching implications since ATP fuels the essential functions of the ER, which include protein folding, quality control, and lipid synthesis. Published in the prestigious journal Nature, the study delineates the critical role of the transporter protein SLC35B1 in channeling ATP into the ER lumen, setting the stage for new therapeutic opportunities targeting diseases linked to ER dysfunction.
At the helm of this research is Professor David Drew from Stockholm University, whose team, in collaboration with researchers across Europe and Japan, has marveled at the intricate molecular choreography governing ATP translocation into the ER. By employing cutting-edge cryo-electron microscopy (cryo-EM), they elucidated high-resolution structures of SLC35B1, visualizing the transporter in multiple conformational states. These structural snapshots have unraveled the mechanistic underpinnings of how SLC35B1 effectively recognizes and facilitates the passage of ATP molecules from the cytosol into the ER interior, a compartment critical for cellular homeostasis.
The ER functions as a cellular nexus, orchestrating the synthesis, folding, and trafficking of proteins and lipids essential for cell survival. These energetically demanding processes rely heavily on ATP, whose precise delivery into the ER has remained an unresolved mystery due to the organelle’s isolation from direct ATP synthesis and cytosolic ATP pools. The confirmation of SLC35B1 as the ATP transporter fills this vital knowledge gap, fundamentally advancing our comprehension of intracellular energy logistics.
Intriguingly, the data show that SLC35B1 operates through a step-wise translocation mechanism, involving specific binding sites that selectively recognize ATP’s physiochemical properties. The cryo-EM structures detail key amino acid residues integral to ATP binding and conveyance, highlighting prospective molecular targets for drug design. By modulating these critical residues, future therapies could influence ATP transport efficiency, offering novel interventions for managing ER stress-related pathologies.
Diseases such as type 2 diabetes, various cancers, and neurodegenerative disorders like Alzheimer’s disease have all been linked to impaired ER function characterized by energy imbalance and protein misfolding. The ability to alter ATP supply within the ER through pharmacological agents targeting SLC35B1 is poised to revolutionize treatment paradigms. Enhanced ATP delivery could restore ER homeostasis in conditions marked by energy deficits, while downregulating transport might suppress aberrant activities in pathological states where ER stress fuels disease progression.
Particularly notable is the interdisciplinary approach behind this advance. Early attempts at identifying the ATP transporter candidate were confounded by conflicting reports and scant biochemical validation. To resolve this, the team leveraged a large-scale CRISPR/Cas9 knockout screening conducted collaboratively with the Giulio Superti-Furga Lab at Austria’s CeMM. This functional genomics approach ranked SLC35B1 among the top five crucial transporters for cellular viability, consolidating its role in ATP transport.
Further experimental validation came from the generation of a highly specific antibody against human SLC35B1 by Norimichi Nomura’s group at Kyoto Medical School. This antibody proved indispensable for stabilizing the transporter protein, effectively increasing its molecular size to a threshold amenable for cryo-EM imaging. Without this step, capturing detailed structural information of such a relatively small membrane protein would have remained elusive, demonstrating the ingenuity behind the methodological advancements.
Professor Drew emphasizes that the uncovered molecular blueprint extends beyond fundamental biology into translational medicine. By revealing SLC35B1’s conformational dynamics and ATP-binding motifs, the study provides a scaffold for the rational design of small molecules capable of fine-tuning transporter activity. Therapeutic modulation could either safeguard ER function by enhancing ATP import in disease states or inhibit it where pathological ER hyperactivity contributes to disease.
Currently, the research consortium is actively screening compound libraries for small molecules that can specifically interact with SLC35B1. These efforts aim to identify candidate molecules capable of modulating ATP transport, thereby paving the way for targeted therapies that rectify ER-related metabolic imbalances. Such drug candidates could usher in a new class of treatments addressing the root causes of ER-associated disorders.
The ramifications of this discovery also resonate with broader cellular physiology, as it sheds light on energy distribution mechanisms within organelles. Understanding how ATP is selectively delivered and consumed within intracellular compartments is a fundamental biological problem with implications across metabolism, signaling, and cell survival pathways. This study places SLC35B1 at the center of this intricate web, providing a tangible target for further exploration.
From a technical standpoint, the study showcases how modern structural biology techniques such as cryo-EM have transformed our ability to visualize membrane proteins in action. By capturing SLC35B1 in multiple functional states, the researchers not only confirm its role but also reveal the dynamic conformational landscape that underpins transporter function. This insight is essential for any future endeavors seeking to manipulate transporter behavior pharmacologically.
Ultimately, this seminal research on SLC35B1 catalyzes a paradigm shift in how we perceive organellar bioenergetics and its linkage to disease. As we deepen our grasp on molecular transport mechanisms, we open avenues to innovative therapeutic strategies that target intracellular energy pathways. The promise of controlling ATP flow within the ER is poised to impact a wide spectrum of diseases where cellular energy dysregulation is pathogenic.
In summary, the identification and detailed characterization of SLC35B1 as the human ER ATP transporter resolves a pivotal question in cell biology and medicine. This work exemplifies the power of multidisciplinary collaboration combining structural biology, biochemistry, genetics, and chemical biology to untangle complex cellular phenomena. With ongoing drug discovery efforts, the path from fundamental discovery to clinical application looks increasingly attainable, heralding a new frontier in combating ER-associated human diseases.
Subject of Research: Cells
Article Title: Step-wise ATP translocation into the ER by human SLC35B1
News Publication Date: 21-May-2025
Web References: https://www.nature.com/articles/s41586-025-09069-w
References: DOI: 10.1038/s41586-025-09069-w
Image Credits: Made by Surabhi Kokane using Biorender.com
Keywords: ATP transport, SLC35B1, endoplasmic reticulum, cryo-electron microscopy, membrane transporter, ER stress, protein folding, cellular bioenergetics, targeted therapy, molecular structure, CRISPR/Cas9 screening, drug discovery
Tags: ATP transport mechanismsbiochemistry research breakthroughscellular energy dynamicscryo-electron microscopy applicationsendoplasmic reticulum functionER dysfunction diseaseshigh-resolution protein structureslipid synthesis processesmolecular transport mechanismsprotein folding and quality controlSLC35B1 protein roletherapeutic targets in cell biology