Gallbladder cancer (GBC) stands as the most prevalent malignancy within the biliary system, notorious for its aggressive nature and propensity for lymph node metastasis. Despite substantial advancements in oncology, the molecular underpinnings orchestrating GBC progression and metastatic dissemination remain largely enigmatic. This knowledge gap significantly hampers the development of targeted therapies, underscoring an urgent need to elucidate the mechanisms driving tumor aggressiveness in GBC. Recently, a groundbreaking study from researchers at Shandong University, published in the journal Genes & Diseases, sheds light on a critical molecular axis that propels GBC metastasis and offers promising avenues for therapeutic intervention.
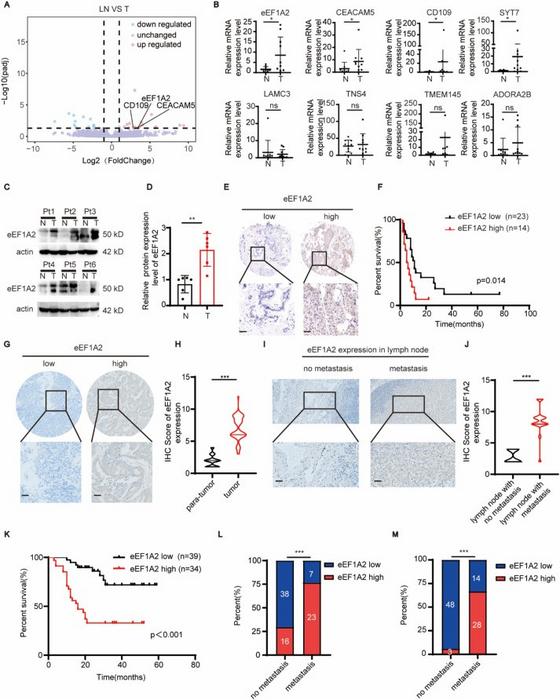
Central to this study is the eukaryotic translation elongation factor 1A2 (eEF1A2), a protein traditionally recognized for its pivotal role in the elongation phase of protein synthesis. Through integrative transcriptomic analyses of matched tumor and metastatic lymph node tissues from gallbladder cancer patients, the investigators identified a pronounced upregulation of eEF1A2 in tumor specimens exhibiting lymph node metastasis. This upsurge in expression correlated robustly with poor patient outcomes, positioning eEF1A2 as a potential biomarker for aggressive disease phenotypes.
An intriguing facet of this study is the revelation that post-translational modifications of eEF1A2, specifically lysine methylation, modulate its oncogenic functions. The researchers identified two critical methylation sites, lysine 36 (K36) and lysine 55 (K55), that are hypermethylated in GBC cells. Focus was placed on the methylation of K36 catalyzed by EEF1AKMT4, a methyltransferase found to be overexpressed in gallbladder tumor tissues. While knockdown of EEF1AKMT4 attenuated malignant behaviors of GBC cells, its overexpression alone did not initiate tumorigenesis, suggesting a nuanced role for this enzyme in tumor biology.
Mechanistic investigations revealed that trimethylation at the K36 residue of eEF1A2 significantly enhances its GTPase activity. This biochemical alteration triggers activation of pro-survival and proliferation signaling pathways, notably ERK1/2 and AKT, through elevated ribosomal protein synthesis. This axis is critical as it underpins the increased biomass and metabolic demands of rapidly proliferating cancer cells. Importantly, restoring EEF1AKMT4 expression in knockdown cells rescues the proliferative, migratory, and invasive phenotypes, underscoring the specificity and functional importance of this methylation event.
This study elegantly uncovers an evolutionarily conserved molecular cascade—the EEF1AKMT4-eEF1A2 K36 trimethylation—ribosomal protein synthesis signaling nexus—that orchestrates gallbladder cancer progression and lymph node metastasis. In the cancer microenvironment, heightened ribosomal output fuels the translation of oncogenic proteins, thus promoting aggressive tumor behavior. Targeting this pathway offers a compelling strategy to stifle tumor growth and dissemination.
Furthermore, immunohistochemical analyses of large GBC patient cohorts reinforced the clinical relevance of eEF1A2 expression patterns. Patients harboring tumors with elevated eEF1A2 levels exhibited significantly worse overall survival rates compared to those with low expression, corroborating its prognostic value. Moreover, a positive correlation between eEF1A2 expression and lymph node metastasis frequency further highlights its role as a facilitator of tumor invasiveness.
This research advances the understanding of the molecular etiology of GBC metastasis, suggesting that therapeutic strategies aimed at interrupting eEF1A2 methylation or its downstream signaling pathways could yield substantial clinical benefits. While the direct inhibitors of eEF1A2 or EEF1AKMT4 remain to be developed, these findings pave the way for future drug discovery endeavors targeting the translational machinery of cancer cells.
Ultimately, the study not only elucidates a novel epigenetic modification that modulates eEF1A2 activity but also places protein synthesis regulation at the heart of gallbladder cancer malignancy. The identification of the EEF1AKMT4-eEF1A2-K36me3-ribosomal protein synthesis axis as a critical driver of tumor progression marks a paradigm shift in comprehending GBC biology and opens new frontiers in cancer therapeutics. As gallbladder cancer continues to present clinical challenges due to late-stage diagnosis and resistance to conventional treatments, such molecular insights offer hope for precision medicine approaches tailored to intercept metastatic cascades.
In conclusion, this seminal work underscores the importance of translation elongation factors beyond their canonical roles, revealing epigenetic regulation as a linchpin in cancer aggressiveness. The demonstration that lysine methylation modulates eEF1A2’s role in GBC implies broader relevance in oncology, inviting studies into similar modifications in other malignancies. Harnessing this knowledge holds promise for devising innovative therapies that can disrupt the symbiotic relationship between cancer growth and protein synthesis machinery, thereby mitigating metastasis and enhancing patient survival outcomes.
Subject of Research: Molecular mechanisms of lymph node metastasis in gallbladder cancer (GBC)
Article Title: EEF1AKMT4-eEF1A2 synergistically facilitates the progression of GBC by promoting ribosomal protein output
Web References:
https://www.sciencedirect.com/journal/genes-and-diseases
https://www.editorialmanager.com/gendis/default.aspx
References: DOI: 10.1016/j.gendis.2025.101619
Image Credits: Genes & Diseases
Keywords: Gallbladder cancer, lymph node metastasis, eEF1A2, EEF1AKMT4, lysine methylation, translation elongation factor, ribosomal protein synthesis, ERK1/2 signaling, AKT pathway, cancer progression, post-translational modification, cancer biomarkers
Tags: biomarkers for aggressive tumorseEF1A2 as a prognostic indicatoreEF1A2 role in cancerfunctional assays in cancer studiesGallbladder cancer progressionlymph node metastasis in GBCmolecular mechanisms of gallbladder cancerprotein synthesis and cancerShandong University cancer researchtargeted therapies for gallbladder cancertherapeutic interventions for GBCtranscriptomic analysis in oncology