In a groundbreaking study that elegantly unravels the intricate relationship between genetic mutations and cellular metabolism, researchers at The Jackson Laboratory have illuminated a novel mechanism by which age-related mutations substantially enhance the proliferative capabilities of blood stem cells. This revelation not only deepens our understanding of how aging primes the body for hematologic disorders but also offers a vivid glimpse into potential therapeutic strategies aimed at halting these pathological expansions before they manifest into serious diseases.
Aging is often accompanied by the subtle and silent accrual of genetic mutations within hematopoietic stem cells—the vital progenitors responsible for replenishing the body’s blood supply. Among these mutations, alterations in the gene Dnmt3a stand out for their frequency and clinical significance. Historically recognized for their marked presence in blood cancers, mutations in Dnmt3a have long puzzled scientists regarding the precise cellular dynamics that enable mutant clones to outcompete their normal counterparts. The recent work led by Jennifer Trowbridge and colleagues offers a highly detailed metabolic perspective that radically advances this narrative.
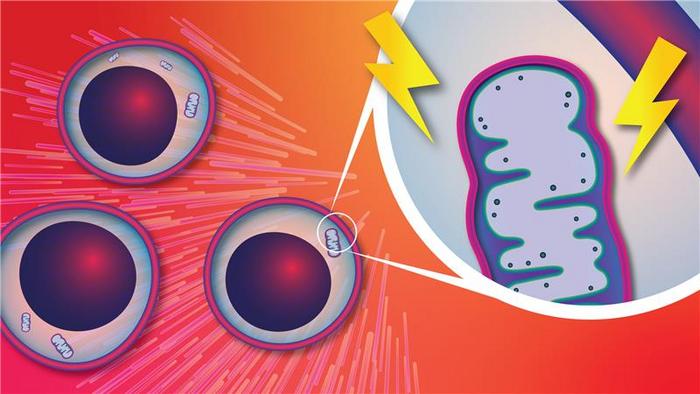
Employing genetically engineered murine models harboring the Dnmt3a mutation, the research team discovered that mutant hematopoietic stem cells exhibit profoundly heightened mitochondrial membrane potential relative to normal cells. This increase in mitochondrial bioenergetic activity translates into a significant elevation of ATP production, likening these mutant stem cells to a biologically “supercharged” engine. Notably, this mitochondrial hyperactivity endows mutant cells with a remarkable growth advantage, allowing them to expand clonally within the bone marrow microenvironment, thereby setting the stage for clonal hematopoiesis.
Clonal hematopoiesis, a condition wherein a substantial fraction of blood cells derive from a single mutated progenitor, is of particular concern due to its strong epidemiological association with age-related diseases such as cardiovascular ailments, hematological malignancies, and inflammatory disorders. The silent nature of clonal expansions renders them especially insidious; many elderly individuals harbor these mutant clones without overt symptoms until disease progression occurs. Deciphering the metabolic adaptations driving this clonal dominance is indispensable for developing targeted interventions.
What makes this study remarkably compelling is the identification of mitochondrial overactivity as an actionable vulnerability within Dnmt3a-mutant stem cells. Through a series of elegant experiments involving mitochondrial-targeting agents such as MitoQ and d-TPP, the researchers demonstrated that these compounds selectively impair mutant cells by disrupting their mitochondrial function and energy production. This specificity spares normal hematopoietic stem cells, which apparently do not depend as heavily on mitochondrial ATP production, underscoring the potential therapeutic window for these agents.
Further elevation of clinical relevance stems from the convergence of these findings with parallel research illustrating that metformin, a widely prescribed anti-diabetic medication known to modulate mitochondrial respiratory chain activity, similarly diminishes the competitive edge of mutant hematopoietic stem cells. This convergence not only validates the mitochondrion as a promising therapeutic target but also raises the tantalizing possibility of repurposing established drugs for the prevention of clonal hematopoiesis and its associated complications.
The mechanistic underpinnings of this bioenergetic shift involve an unexpected linkage between an epigenetic regulator, Dnmt3a, and mitochondrial metabolism, a connection hitherto unappreciated in the field. Traditionally, Dnmt3a has been characterized as a DNA methyltransferase that influences gene expression epigenetically; however, this study uncovers its indirect yet profound impact on mitochondrial function, reshaping the conceptual framework of how genetic mutations can confer metabolic advantages to stem cells.
These discoveries carry profound implications for the future of precision medicine in geriatric hematology. By illuminating a metabolic Achilles heel in mutant blood stem cells, researchers have paved the way for therapeutic strategies aimed not merely at symptom management but at disease interception. Early intervention in individuals harboring Dnmt3a mutations might, therefore, prevent the emergence of severe blood cancers and reduce the burden of cardiovascular disease linked to clonal hematopoiesis.
It is pertinent to recognize that while these results illuminate new paths, they also prompt essential questions regarding the broader applicability of mitochondrial-targeting therapies. The heterogeneity of mutations driving clonal hematopoiesis means that each genetic variant might exploit distinct metabolic pathways for growth advantage. Future investigations will need to delineate whether mitochondrial dependency is a universal feature or unique to certain mutations such as Dnmt3a.
Moreover, understanding the long-term effects of manipulating mitochondrial function in stem cells is critical. Mitochondria serve as dynamic hubs regulating numerous cellular processes beyond energy production, including apoptosis, reactive oxygen species generation, and signaling. Therapeutic interventions must therefore balance eradicating pathogenic clones while preserving stem cell viability and function.
In parallel, the translation of these findings to human patients appears highly promising. The team successfully demonstrated that human hematopoietic stem cells engineered to carry DNMT3A mutations similarly respond to mitochondrial inhibitors, manifesting decreased proliferative advantage and normalized metabolic activity. This cross-species validation sets a strong precedent for clinical exploration of mitochondrial-targeting agents in managing clonal hematopoiesis.
Ultimately, the synergy between genetic, epigenetic, and metabolic research exemplified in this study represents a paradigm shift in our approach to aging-related blood disorders. By targeting the metabolic lifelines sustaining mutant stem cells, scientists move closer to intercepting diseases at their preclinical origins, potentially transforming patient outcomes across multiple domains where clonal hematopoiesis exerts detrimental effects.
This research also highlights the importance of integrative scientific approaches that combine molecular biology, genetics, metabolism, and pharmacology. Such interdisciplinary synergy is indispensable for unraveling the complexities of stem cell biology and age-associated diseases. As we continue to decode the multifaceted network of cellular aging, new therapeutic horizons like mitochondrial modulation are poised to redefine modern medicine’s capacity to combat age-related pathologies.
Harnessing these insights, clinical trials designed to test mitochondrial inhibitors and metabolic modulators in older populations with detectable clonal hematopoiesis could revolutionize preventive care. These efforts would represent a proactive shift from reactive treatment of advanced disease to precision-based prophylaxis, thereby reducing morbidity and mortality linked with hematological and cardiovascular sequelae of aging.
Subject of Research: Animals
Article Title: Elevated mitochondrial membrane potential is a therapeutic vulnerability in Dnmt3a-mutant clonal hematopoiesis
News Publication Date: 16-Apr-2025
Web References:
https://www.nature.com/articles/s41467-025-57238-2
https://www.nature.com/articles/s41586-025-08871-w
Image Credits: The Jackson Laboratory
Keywords: Stem cell research, Stem cell therapy, Cancer stem cells, Hematopoietic stem cells, Mutation, Heart disease, Blood cancer, Drug therapy, Disease prevention
Tags: aging-related blood disordersblood stem cell dynamicsDnmt3a gene mutations and blood cancergenetic mutations and cellular metabolismhematopoietic stem cell proliferationimplications of mitochondrial function in agingJackson Laboratory hematology studymitochondrial bioenergetics in stem cellsresearch on aging and genetic mutationsrole of mitochondria in agingtherapeutic strategies for hematologic disordersunderstanding age-related mutations