Glioblastoma multiforme (GBM) stands as an exceedingly aggressive brain tumor, characterized by its high malignancy and dismal prognosis. Researchers have long sought effective therapeutic targets, but the complex biology of GBM has hindered significant advancements in treatment. With the rise of personalized medicine, understanding the molecular mechanisms underlying tumor progression is becoming increasingly valuable. Recent findings regarding high-mobility group nucleosomal-binding domain 2 (HMGN2) shed light on new avenues for therapeutic intervention in glioblastoma.
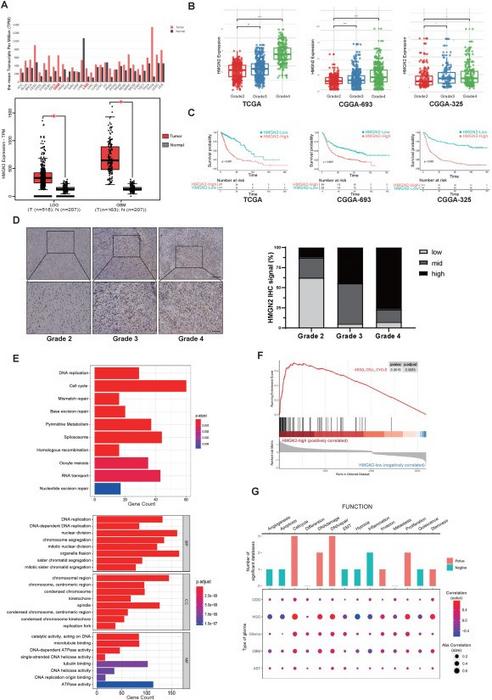
Emerging from a collaborative investigation by scientists from Chongqing Medical University and Southwest University, this study explored the role of HMGN2 in glioma, providing a comprehensive bioinformatics analysis that exposed a significant upregulation of HMGN2 expression across various glioma subtypes. Notably, the expression levels of this protein correlate positively with glioma grades. Low-grade gliomas exhibited lower levels of HMGN2, while high-grade gliomas displayed a marked increase, suggesting a potential biomarker for tumor aggressiveness.
The research hinges on data sourced from extensive cancer genomics databases, including The Cancer Genome Atlas (TCGA) and the Chinese Glioma Genome Atlas (CGGA). Employing mRNA sequencing techniques, researchers pinpointed a vital association between HMGN2 and cell cycle regulation, implicating its role in promoting the proliferative capacity of glioma cells. This growing body of evidence illustrates how HMGN2 may facilitate glioma progression, further underscoring the need for targeted therapies that inhibit this oncogenic pathway.
In terms of mechanistic insights, the study delved into the epigenetic influences exerted by HMGN2. Subsequent investigations revealed that HMGN2 interacts with histones, enhancing the stability of acetylation at critical promoter regions, specifically in the CDC20 gene. This epigenetic modification augments the transcriptional activity of CDC20, a crucial regulator of the cell cycle, thereby promoting glioma cell proliferation. The relationship delineates a sophisticated regulatory axis whereby HMGN2 manipulates cell cycle dynamics to fuel tumor growth.
Knockdown experiments further elucidated the functional importance of HMGN2 in glioma biology. When HMGN2 expression was diminished, researchers noted a significant downregulation of CDC20, accompanied by a consequent reduction in cell proliferation. Conversely, selective restoration of CDC20 expression during rescue experiments reinstated glioma proliferation rates while altering the population dynamics of cells transitioning through the G2/M phase of the cell cycle. These findings demonstrate how tightly interwoven the roles of HMGN2 and CDC20 are within glioma cellular mechanisms.
Clinical relevance emerged as another pivotal aspect of the study, establishing a correlation between CDC20 expression and patient survival outcomes within glioma cohorts. The negative correlation suggests that elevated levels of CDC20 may be indicative of poorer prognoses, simultaneously reinforcing HMGN2’s position as a potential therapeutic target in a clinical setting. This association emphasizes the need for comprehensive analyses to facilitate risk stratification among glioma patients, ultimately leading to more personalized treatment strategies.
In summary, the elucidation of the HMGN2/CDC20 axis reveals a critical pathway in glioma progression, highlighting its potential as a target for novel therapeutic interventions. By manipulating epigenetic regulators such as HMGN2, future therapies could offer new hope for glioblastoma patients facing limited treatment options. The study contributes significantly to the growing understanding of molecular pathogenesis in gliomas, paving the way for innovative research directions aimed at improving patient outcomes and survival rates in aggressive brain tumors.
As we reflect on this research, it’s essential to recognize the broader implications for glioma treatment paradigms. The findings advocate for further investigation into HMGN2 as a prospective biomarker and therapeutic target. They underscore the necessity of integrating molecular insight into clinical practice, enriching our approach to combating glioblastoma through enhanced precision medicine strategies. This underscores the promise of translating complex molecular findings into tangible therapeutic opportunities, consequently reshaping the landscape of glioma treatment while addressing the persistent challenges posed by this malignant condition.
In conclusion, the continual exploration of the role of genomic and epigenomic factors in glioblastoma will remain pivotal. Future research should focus on the development of targeted therapies that can effectively disrupt the HMGN2/CDC20 regulatory pathway. By leveraging such insights, we strive to transition from traditional treatment methodologies to more personalized, molecularly-informed approaches, ultimately reducing mortality associated with glioblastoma and improving the quality of life for affected individuals.
Subject of Research: Glioblastoma and the role of HMGN2 in cancer progression.
Article Title: HMGN2 accelerates the proliferation and cell cycle progression of glioblastoma by regulating CDC20 expression.
News Publication Date: October 2023.
Web References: ScienceDirect
References: Genes & Diseases Journal
Image Credits: Genes & Diseases
Keywords: Glioblastoma, HMGN2, CDC20, cancer progression, epigenetics, cell cycle, therapeutic targets, molecular research.
Tags: cancer genomics databasescell cycle regulation in tumorsglioblastoma multiforme prognosisglioma grade correlationglioma molecular mechanismsglioma treatment advancementshigh-mobility group nucleosomal-binding domain 2HMGN2 as a biomarkermRNA sequencing in glioma researchpersonalized medicine in oncologyTCGA and CGGA studiestherapeutic targets for gliomas