Covalent bonding is a fundamental principle in chemistry, known for uniting atoms through shared electron pairs. However, within the natural world, the dynamics are far more intricate, with molecules often interlinked through weaker, yet equally significant, forces that form supramolecular networks. These structures can emerge spontaneously from initial molecular groupings, evolving into expansive and stable architectures. The study of these networks is not just an academic endeavor; they play pivotal roles in the very fabric of biological systems.
A striking example of supramolecular networks in action is observed in the cellular mechanisms governing nutrient uptake. Cells utilize hexagonal supramolecular networks, formed from the three-armed protein clathrin, to encapsulate and internalize nutrients. The clathrin-driven networks create vesicular structures around nutrients, effectively transporting them into the cell. Notably, another protein, TRIM5a, assembles into a hexagonal lattice around HIV viruses, thwarting their replication process. This affinity for hexagonal formations spans various scales and contexts, manifesting prominently in nature’s architecture — think of the classic hexagonal patterns found in beehives.
This intriguing relationship between structure and function in biological systems has driven researchers at the École Polytechnique Fédérale de Lausanne (EPFL) to probe deeper into the mechanics of these networks. In a groundbreaking study published in Nature Chemistry, a collaborative team from the Programmable Biomaterials Lab (PBL) and the Laboratory for Bio- and Nano-Instrumentation (LBNI), led by Georg Fantner, embarked on an exploration of crystalline supramolecular network formation using nanoscale DNA strands designed in a three-point star configuration. Their investigations unveiled critical parameters that govern this process, emphasizing a key factor that supersedes traditional understandings of bond strength.
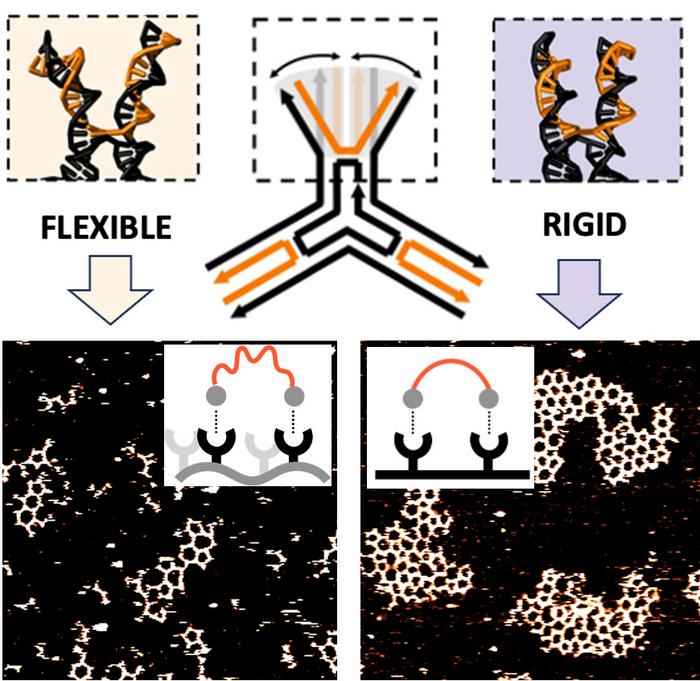
The researchers identified a novel concept known as “interface flexibility,” which emerged as a decisive variable in the stability and organization of supramolecular networks. Much like the genetic material in living organisms, the three-point star DNA molecules were varied in their nucleotide sequences. These variations influenced how strongly the molecules interacted with adjoining units. However, the study introduced an additional layer of complexity—by meticulously adjusting the lengths of the strands constituting the arms of the DNA stars, the researchers could manipulate both local and global flexibility.
Advanced imaging techniques, particularly high-speed atomic force microscopy, revealed striking differences in network formation based on the rigidity of these arms. DNA stars equipped with shorter and stiffer arms were observed to seamlessly organize into stable hexagonal networks. In contrast, those with elongated, more pliable arms struggled to establish substantial networks, often leading to a lack of coherence in structure. Computational simulations lent further credence to these findings, demonstrating that the shorter arms had a near fourfold increase in the likelihood of parallel alignment, which is crucial for effective connection with neighboring molecules. Meanwhile, the longer arms risked spreading too far apart, inhibiting the formation of stable interactions.
Bastings emphasized this revolutionary insight, stating that the interface where two molecules converge must exhibit rigidity. If one of the participating molecules demonstrates flexibility, the probability of maintaining a robust connection diminishes dramatically. This concept challenges prevailing theories, advocating that interface flexibility, rather than binding strength, plays a predominant role in the successful formation of supramolecular networks.
Moreover, the research team uncovered an intriguing capability to fine-tune interface flexibility. By applying targeted changes to flexible molecules, they could enhance local rigidity precisely at the binding interface, fostering network growth while preserving the overall dimensions of the molecular structures. This revelation paves the way for designing semi-flexible monomers that retain their propensity for self-assembly by controlling interfaces effectively.
The implications of this research extend far beyond theoretical exploration. Bastings envisions transformative potential in the design of proteins and other molecular assemblies for applications in self-assembly processes. An informed design strategy focused on inducing local rigidity could facilitate the creation of new supramolecular networks, which could be harnessed for targeted cellular nanotherapies. Conversely, this understanding could also enable the intentional induction of flexibility to disrupt or prevent the formation of undesirable networks, such as amyloid plaques associated with neurodegenerative diseases like Alzheimer’s.
In addition to biological applications, the insights gained from this study could revolutionize fields like spintronics. The precise engineering of nanoscale networks could significantly enhance the development of next-generation electronics, tapping into the self-assembly properties of well-defined molecular structures. The confluence of diverse scientific disciplines has played a crucial role in the success of this research effort, illustrating the power of collaboration and innovation.
This study stands as a testament not only to the ingenuity of the researchers involved but also to the fundamental versatility of DNA. Bastings acknowledged the role of interdisciplinary DNA nanotechnology and advancements in atomic-level property control, which have enabled the extraction of DNA from its traditional genomic context. By leveraging its inherent properties, researchers can probe a myriad of physical interactions, offering exciting prospects for future endeavors in molecular science.
As we continue to unravel the layers of molecular interaction and network formation, this research sheds light on the intricate dance of flexibility and rigidity that governs the assembly of supramolecular structures. The findings underscore the importance of reconsidering longstanding assumptions in molecular dynamics and highlight the exciting horizon ahead for applications in health, materials science, and beyond.
Subject of Research: Supramolecular networks and their formation dynamics
Article Title: Interface flexibility controls the nucleation and growth of supramolecular networks
News Publication Date: 13-Feb-2025
Web References: Not applicable
References: Not applicable
Image Credits: © PBL EPFL
Keywords: Supramolecular networks, DNA nanotechnology, interface flexibility, protein design, self-assembly, molecular interactions, biomedical applications, spintronics, atomic-level control, clathrin networks, TRIM5a, amyloid plaques.
Tags: advancement in chemistry and biologybiological implications of molecular architectureclathrin protein and nutrient uptakecovalent bonding and molecular interactionsdynamics of molecular groupingsEPFL research on molecular networkshexagonal lattices in cellular mechanismsmolecular network formationsignificance of supramolecular networkssupramolecular structures in biologyTRIM5a and HIV replicationweak forces in molecular bonding