A groundbreaking study conducted by researchers from Mount Sinai and Memorial Sloan Kettering Cancer Center has illuminated the intricate relationship between histone monoaminylation dynamics and circadian gene expression. This collaborative research has unveiled a previously unrecognized mechanism by which chemical bonding of monoamine neurotransmitters—specifically serotonin, dopamine, and histamine—affects the regulation of brain physiology and behavior through their modifications of histone proteins, which are critical for DNA packaging in our cells.
This pioneering work has significant implications for understanding how fluctuations in neurotransmitter levels can influence circadian rhythms. By elucidating the role of monoamines in histone modifications, the research team has opened new avenues for developing targeted therapies for conditions characterized by circadian rhythm disruptions such as insomnia, mood disorders like depression and bipolar disorder, and neurodegenerative diseases.
Lead author Ian Maze, PhD, a prominent figure in the field of neuroscience at the Icahn School of Medicine, articulated the importance of their findings: the study highlights that the brain’s internal clock is influenced directly by chemical neurotransmitters in a manner that alters gene expression. The dynamic interplay of these monoamines modifies histones, thus regulating essential patterns of circadian gene expression and neuronal plasticity that dictate sleep and wakefulness.
Adding to the significance of this study, co-lead author Yael David, PhD, emphasized the revelation of how circadian events can elicit neurotransmitter signaling, and vice versa, thereby affecting neuronal dynamics through modifications in DNA structure. The complexity of these interactions underscores the need for a deeper exploration of these mechanisms to contribute toward developing therapeutic strategies for treating various brain disorders.
A critical aspect of this research lies in prior work conducted by the Maze Lab, which revealed how serotonin and dopamine not only function as essential neurotransmitters but also attach to specific histone proteins (H3) to modify gene expression programs. Dysregulation of these processes could lead to adverse behavioral and biological outcomes, including susceptibility to stress and potential drug relapse, illustrating the vital role that histone modifications play in our mental health.
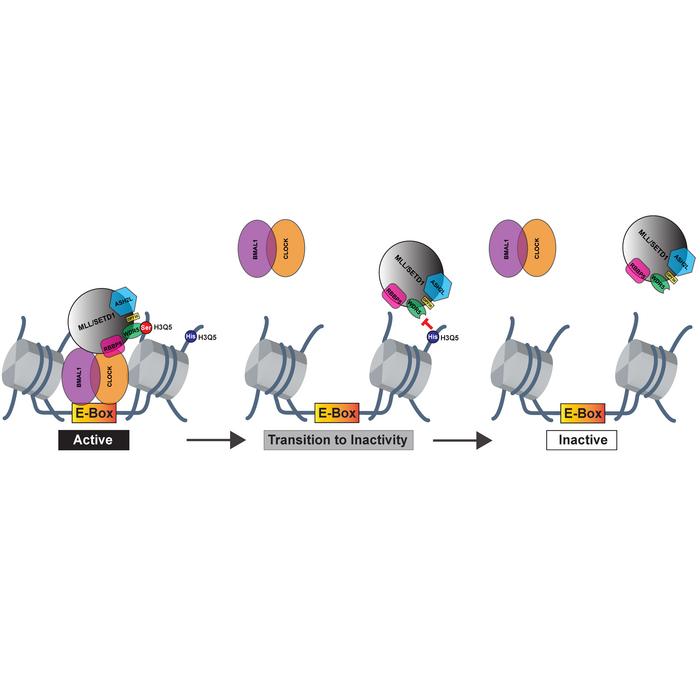
Throughout the study, researchers utilized a highly interdisciplinary approach, employing various experimental techniques to decode the biochemical mechanisms of transglutaminase 2 (TG2), the enzyme responsible for histone monoaminylation. The novel findings showed TG2’s dual function: it not only adds monoamines to histone H3 but also has the capability to erase or exchange one monoamine for another, facilitating complex gene expression control through distinct mechanisms.
The observation that different brain regions containing heterogeneous pools of monoamines might rapidly exchange these neurotransmitters on histones was a key highlight. This rapid exchange in response to external stimuli enables intricate regulation of gene expression programs that could be pivotal in regulating various neural activities.
The discovery of histaminylation as a new modification of histones marks a significant milestone in understanding these processes. This modification, along with H3 serotonylation, was shown to play an essential role in regulating circadian rhythm and behavior in murine models, indicating a fascinating layer of neurotransmission-independent mechanisms governing sleep/wake cycles.
Histamine’s involvement extends beyond mere neurotransmission; its role in various biological processes and disease states adds to the urgency of this research. With implications for immune system regulation and potential connections to cancer, understanding how TG2-dependent monoaminylation of histones is controlled may shed light on several health conditions characterized by monoaminergic dysregulation.
By shedding light on TG2’s regulatory mechanisms, researchers anticipate significant insights into diseases such as depression, schizophrenia, and Parkinson’s disease. The foundational aspects of this study pave the way for future investigations that could lead to innovative therapeutic approaches, enhancing our understanding of circadian biology and mental health interventions.
As the research community moves forward, the collective efforts invested in recognizing these biochemical pathways raise essential questions regarding circadian regulation and its implications for health. The study elegantly encapsulates the unexpected ways in which our brains integrate complex biochemical signals to orchestrate essential functions like sleep and wakefulness.
The findings reveal an exciting frontier in neuroscience, where the molecular intricacies of neurotransmitter modifications furnish deeper insights into how we can harness these mechanisms for therapeutic benefit. This research underscores a pivotal step in bridging the gap between basic research and clinical application, ultimately inspiring hope for improved treatment strategies for conditions intertwined with circadian rhythms.
The collaborative efforts of the Mount Sinai and Memorial Sloan Kettering teams not only enrich our scientific understanding but also represent a significant advancement in delineating the molecular nexus of neurotransmitter actions in the brain, setting the stage for future breakthroughs in neuroscience and therapeutic innovations.
Subject of Research: Animals
Article Title: Bidirectional histone monoaminylation dynamics regulate neural rhythmicity
News Publication Date: 8-Jan-2025
Web References: Nature
References: DOI 10.1038/s41586-024-08371-3
Image Credits: Credit: Benjamin Weekley, PhD, Icahn School of Medicine at Mount Sinai
Keywords: Histone modification, Histones, Neural mechanisms, Regulatory mechanisms, Intracellular proteins, Biological rhythms.