Infant gut microbiomes oscillate with a circadian rhythm, even when they are cultivated outside of the body. Researchers report April 2 in the journal Cell Host & Microbe that the rhythm is detectable as early as 2 weeks after birth but becomes more pronounced with age. The finding comes from a randomized controlled trial that also showed that diet has less impact on the development and composition of the infant microbiome than previously thought.
Credit: Cell Host & Microbe/Heppner et al.
Infant gut microbiomes oscillate with a circadian rhythm, even when they are cultivated outside of the body. Researchers report April 2 in the journal Cell Host & Microbe that the rhythm is detectable as early as 2 weeks after birth but becomes more pronounced with age. The finding comes from a randomized controlled trial that also showed that diet has less impact on the development and composition of the infant microbiome than previously thought.
“We found that even at very early ages of colonization, the microbial ecosystem develops this circadian rhythmicity,” says senior author and microbiome expert Dirk Haller of the Technical University of Munich. “We have shown these rhythms before in adults, but we were not sure when these mechanisms first appear.”
While diet had only a marginal impact on infant microbiome development, the researchers showed that age plays a more important role.
“Diet matters, but less than aging of the gut,” says Haller. “When we compared breastfed and formula-fed infants, the differences in microbiome colonization were marginal. Our intestinal system is probably a little bit more flexible in adapting to what the environment has to offer.”
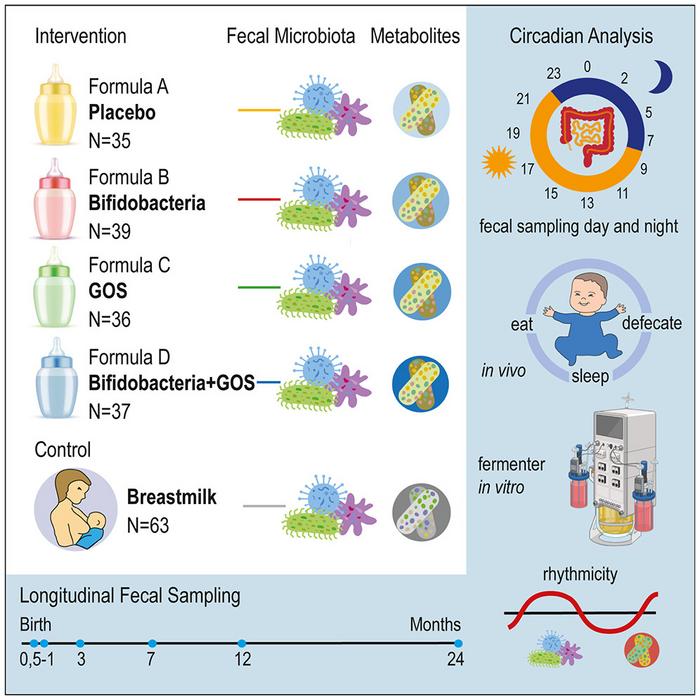
The researchers used a randomized controlled trial to compare microbiome development in infants that were exclusively breastfed with infants who received different types of formula—un-supplemented formula; formula containing breast-milk-derived bacteria (Bifidobacteria); formula containing breast-milk-mimicking sugars (galacto-oligosaccharides, GOSs); or formula containing both Bifidobacteria and GOSs. Altogether, the trial included 210 infants.
To longitudinally track the infants’ microbiomes, the team sampled the infants’ stools when they were 0.5 months, 1 month, 3 months, 7 months, and 12 months of age as well as at 24 months for a subset of the infants. They also kept note of the time of day that the stool sample was collected.
The researchers found that diet had little impact on infant growth or the differences in the infants’ microbiomes. Though there was a lot of variation, all of the infants showed a gradual increase in gut microbe diversity, and at 24 months there was no observable difference between the groups. When they compared the different types of formula, they found that GOS-supplemented infant formula was more effective at promoting sustained levels of Bifidobacteria compared to formula containing Bifidobacteria.
However, there was a significant difference in the gut metabolite profile between exclusively breastfed and formula-fed infants. “The metabolite environment in the gut is dramatically different between a baby that is exclusively breastfed and babies that receive infant formula, which could have a fundamental impact on metabolic priming and many downstream effects,” says Haller. “We can conclude that breast milk does something completely different in the metabolism in in the infant’s intestine.”
The researchers also observed rhythmic 24-hour fluctuations in the abundance of different microbiome species. When they took infant microbes and grew them in continuous culture in the lab, the bacteria settled into the same circadian rhythm—even in the absence of external light or host cues. Though circadian rhythms have been previously observed in adult microbiomes, this is the first evidence that bacteria maintain these rhythms independently.
“When we take them out, they maintain these daytime-related diurnal oscillations,” Haller says. “This is fairly surprising because it suggests that the bacteria have some intrinsic mechanism that provides some sort of adaptation to a day and night cycle, which could potentially give them an advantage in colonizing the human intestine.”
The researchers plan to further investigate microbiome circadian rhythms in future studies. Specifically, they want to examine whether individual bacterial species maintain rhythms when grown in isolation rather than in complex communities and to search for the genes that control these rhythms.
“For us, the next question is can we identify mechanisms in bacteria that control their circadian behavior,” says Haller.
###
This research was supported by Töpfer GmbH, the German Research Foundation, the Joint Programming Initiative of the European Union, and the German Ministry of Education and Research.
Cell Host & Microbe, Heppner et al., “Diurnal rhythmicity of infant fecal microbiota and metabolites: A randomized controlled interventional trial with infant formula” https://cell.com/cell-host-microbe/fulltext/S1931-3128(24)00058-1
Cell Host & Microbe (@cellhostmicrobe), published by Cell Press, is a monthly journal that publishes novel findings and translational studies related to microbes (which include bacteria, fungi, parasites, and viruses). The unifying theme is the integrated study of microbes in conjunction and communication with each other, their host, and the cellular environment they inhabit. Visit http://www.cell.com/cell-host-microbe. To receive Cell Press media alerts, contact [email protected].
Journal
Cell Host & Microbe
DOI
10.1016/j.chom.2024.02.015
Method of Research
Experimental study
Subject of Research
People
Article Title
Diurnal rhythmicity of infant fecal microbiota and metabolites: a randomized controlled interventional trial with infant formula
Article Publication Date
2-Apr-2024