In our technologically dependent society, the mobility, dependability and safety of our devices, including phones and laptops, is critical. Just as important is our ability to easily charge and recharge these devices so they are available when we need them. To do this, we use rechargeable batteries, specifically lithium-ion batteries (LIB). They give us the freedom of movement and connectivity we need. As society’s needs evolve, so too does our tech, and so too must the batteries that allow us to use this tech. One of the most urgent concerns regarding lithium-ion batteries is their safety. Though rare, there are issues with explosions and fires caused by electrochemical system instability. “Consequently, there is an urgent need to develop LIBs that can provide higher energy density, longer cycle life and improved safety,” said Ying Bai, corresponding author of the paper and a professor at Beijing Institute of Technology in China.
Credit: Energy Materials and Devices, Tsinghua University Press
In our technologically dependent society, the mobility, dependability and safety of our devices, including phones and laptops, is critical. Just as important is our ability to easily charge and recharge these devices so they are available when we need them. To do this, we use rechargeable batteries, specifically lithium-ion batteries (LIB). They give us the freedom of movement and connectivity we need. As society’s needs evolve, so too does our tech, and so too must the batteries that allow us to use this tech. One of the most urgent concerns regarding lithium-ion batteries is their safety. Though rare, there are issues with explosions and fires caused by electrochemical system instability. “Consequently, there is an urgent need to develop LIBs that can provide higher energy density, longer cycle life and improved safety,” said Ying Bai, corresponding author of the paper and a professor at Beijing Institute of Technology in China.
Beijing scientists have been researching the use of additives in the sulfone-based electrolyte of lithium-ion batteries to improve their performance. They found that by adding triphenylphosphine oxide (TPPO), “that the TPPO improves the thermal stability of the electrolyte, which has important industrial value and foundational significance of TPPO as an additive for advancing the development of LIB’s,” said Chuan Wu, co-corresponding author on the paper and a professor at Beijing Institute of Technology.
The results of their research will be published in Energy Materials and Devices on January 30.
When lithium-ion batteries are discharging lithium-ions, they move from an anode, which is an electrode where current enters the battery, through an electrolyte pass through a separator to a cathode, which is where the current leaves the storage battery to energize a device. The path is reversed when recharging. “In the composition of the battery, the non-aqueous electrolyte used in LIBs plays a crucial role in determining key performance parameters such as cycle life, power density and efficiency,” said Ying Bai. Power density is a measure of stored power per volume and cycle life is the number of charge/discharge cycles that a battery can undergo before it starts to decrease the percentage of charge it can hold.
The electrolyte solutions in use now have some issues with cycle stability, thermal stability and safety. Rather than completely changing the electrolyte solution, they chose to test the use of an additive, TPPO, in the electrolyte to improve the performance of the overall battery.
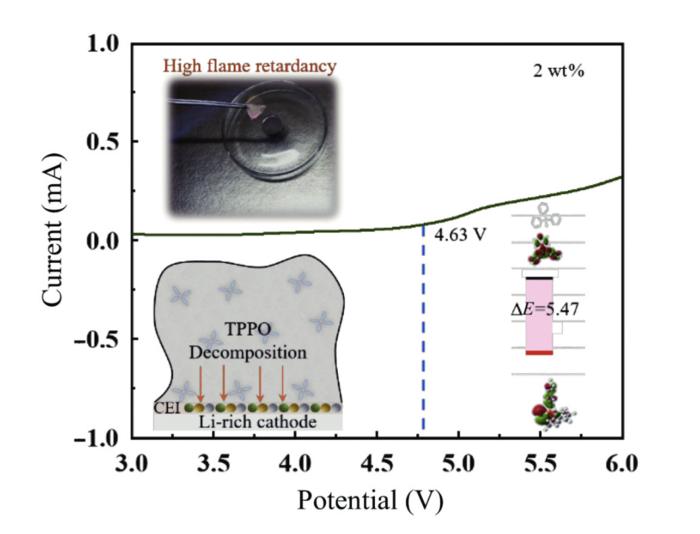
TPPO when tested was found to have several important properties. “Firstly, it reduces the flame point of the sulfone electrolyte; Secondly, it selectively forms a stable passivation film, enhancing the interface stability between the sulfone electrolyte and the electrode material,” said Chuan Wu. The passivation film forms as the TPPO decomposes and coats the cathode, rendering it more resistant to wear and tear, similarly reducing the breakdown of the electrolyte while enhancing the movement of the lithium-ions across the electrolyte.
Using theoretical calculations, electrochemical characterization and flammability tests, the researchers found “that the addition of 2 wt.% TPPO to the sulfone-based electrolyte significantly enhances the ionic conductivity within the temperature range of 20–60°C. Additionally, it increases the discharge capacity of LIBs in the range of 2–4.8 V while maintaining excellent rate performance and cycling stability. Flammability tests and thermal gravimetric analysis (TGA) results indicate the excellent non-flammability and thermal stability of the electrolyte,” said Ying Bai.
In short, the new electrolyte that they have developed is safer as it is non-flammable, is thermally stable and has an increased energy discharge capacity.
Other contributors include Qiaojun Li, Wenya Wu, Haixia Ren from the School of Materials Science and Engineering, Beijing Institute of Technology, Beijing, China; Yu Li from the School of Materials Science and Engineering, Beijing Institute of Technology, Beijing, China, and Yangtze Delta Region Academy of Beijing Institute of Technology, Jiaxing, China.
This research was supported by the National Key Research and Development Program of China, the Science and Technology Program of Guangdong Province, funding from General Research Institute for Nonferrous Metals and the Beijing Institute of Technology Research Fund Program for Young Scholars.
About Energy Materials and Devices
Energy Materials and Devices is launched by Tsinghua University, published quarterly by Tsinghua University Press, aiming at being an international, single-blind peer-reviewed, open-access and interdisciplinary journal in the cutting-edge field of energy materials and devices. It focuses on the innovation research of the whole chain of basic research, technological innovation, achievement transformation and industrialization in the field of energy materials and devices, and publishes original, leading and forward-looking research results, including but not limited to the materials design, synthesis, integration, assembly and characterization of devices for energy storage and conversion etc.
About SciOpen
SciOpen is a professional open access resource for discovery of scientific and technical content published by the Tsinghua University Press and its publishing partners, providing the scholarly publishing community with innovative technology and market-leading capabilities. SciOpen provides end-to-end services across manuscript submission, peer review, content hosting, analytics, and identity management and expert advice to ensure each journal’s development by offering a range of options across all functions as Journal Layout, Production Services, Editorial Services, Marketing and Promotions, Online Functionality, etc. By digitalizing the publishing process, SciOpen widens the reach, deepens the impact, and accelerates the exchange of ideas.
Journal
Energy Materials and Devices
DOI
10.26599/EMD.2023.9370022
Article Title
Enhanced safety of sulfone-based electrolytes for lithium-ion batteries: Broadening electrochemical window and enhancing thermal stability
Article Publication Date
30-Jan-2024




