“The present studies were performed to extend our knowledge of GZ17-6.02 biology from that known in solid tumor cell types such as prostate cancer cells to liquid tumor cell types, for example, mycosis fungoides.”
Credit: 2024 Booth et al.
“The present studies were performed to extend our knowledge of GZ17-6.02 biology from that known in solid tumor cell types such as prostate cancer cells to liquid tumor cell types, for example, mycosis fungoides.”
BUFFALO, NY- February 26, 2024 – A new research paper was published in Oncotarget’s Volume 15 on February 8, 2024, entitled, “GZ17-6.02 interacts with bexarotene to kill mycosis fungoides cells.”
In this new study, researchers Michael R. Booth, Laurence Booth, Jane L. Roberts, Cameron West, and Paul Dent from Virginia Commonwealth University and Genzada Pharmaceuticals investigated the therapeutic agent GZ17-6.02, composed of curcumin, harmine and isovanillin.
“Combined with our curcumin findings, we believe that isovanillin can complex with curcumin and harmine to create an entity with unique biology when compared to the three individual agents.”
GZ17-6.02 has undergone phase I evaluation in patients with solid tumors (NCT03775525) with an RP2D of 375 mg PO BID. The biology of GZ17-6.02 in malignant T cells and in particular those derived from mycosis fungoides (MF) patients, has not previously been studied. The researchers found that GZ17-6.02 alone and in combination with standard-of-care agents was effective in killing MF cells.
“All three components are necessary for optimal killing of MF cells.”
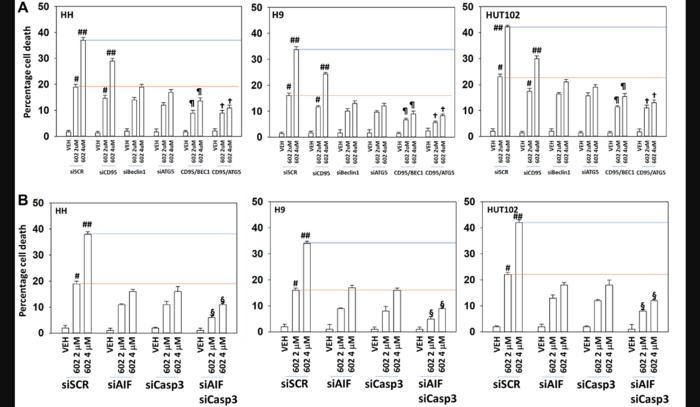
GZ17-6.02 activated ATM, the AMPK, NFκB and PERK and inactivated ERK1/2, AKT, ULK1, mTORC1, eIF2α, and reduced the expression of BCL-XL and MCL1. GZ17-6.02 increased ATG13 S318 phosphorylation and the expression of Beclin1, ATG5, BAK and BIM. GZ17-6.02 in a dose-dependent fashion enhanced autophagosome formation and autophagic flux, and tumor cell killing.
Signaling by ATM and AMPK were both required for efficient killing but not for the dose-response effect whereas ER stress (eIF2α) and macroautophagy (Beclin1, ATG5) were required for both efficient killing and the dose-response. Knock down of the death receptor CD95 reduced killing by ~20% and interacted with autophagy inhibition to further reduce killing, collectively, by ~70%. Inhibition of autophagy and knock down of death-mediators downstream of the mitochondrion, AIF and caspase 3, almost abolished tumor cell killing. Hence in MF cells, the team wrote that GZ17-6.02 is a multi-factorial killer, utilizing ER stress, macroautophagy, death receptor signaling and directly causing mitochondrial dysfunction.”
“We discovered that GZ17-6.02 containing harmine, isovanillin and curcumin caused more tumor cell killing than any of the agents individually or in pairs, and that it could interact in an additive fashion with standard of care MF drugs such as bexarotene and vorinostat to cause additional tumor cell death.”
Read the full paper: DOI: https://doi.org/10.18632/oncotarget.28557
Correspondence to: Paul Dent
Email: [email protected]
Keywords: autophagy, ER stress, GZ17-6.02, bexarotene, vorinostat
Click here to sign up for free Altmetric alerts about this article.
About Oncotarget: Oncotarget (a primarily oncology-focused, peer-reviewed, open access journal) aims to maximize research impact through insightful peer-review; eliminate borders between specialties by linking different fields of oncology, cancer research and biomedical sciences; and foster application of basic and clinical science.
To learn more about Oncotarget, visit Oncotarget.com and connect with us on social media:
- X, formerly Twitter
- YouTube
- Spotify, and available wherever you listen to podcasts
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact [email protected].
Oncotarget Journal Office
6666 East Quaker Street., Suite 1A
Orchard Park, NY 14127
Phone: 1-800-922-0957 (option 2)
###
Journal
Oncotarget
DOI
10.18632/oncotarget.28557
Method of Research
Experimental study
Subject of Research
People
Article Title
GZ17-6.02 interacts with bexarotene to kill mycosis fungoides cells
Article Publication Date
8-Feb-2024