The scientists who received the 2017 Nobel Prize in chemistry were honored for their development of a technique called cryo-electron microscopy, or cryo-EM. The technology was revolutionary because it enabled scientists to see the atomic structure of biological molecules in high resolution.
Credit: Roger Castells-Graells/UCLA
The scientists who received the 2017 Nobel Prize in chemistry were honored for their development of a technique called cryo-electron microscopy, or cryo-EM. The technology was revolutionary because it enabled scientists to see the atomic structure of biological molecules in high resolution.
But cryo-EM still had a catch: It was only effective for imaging large molecules.
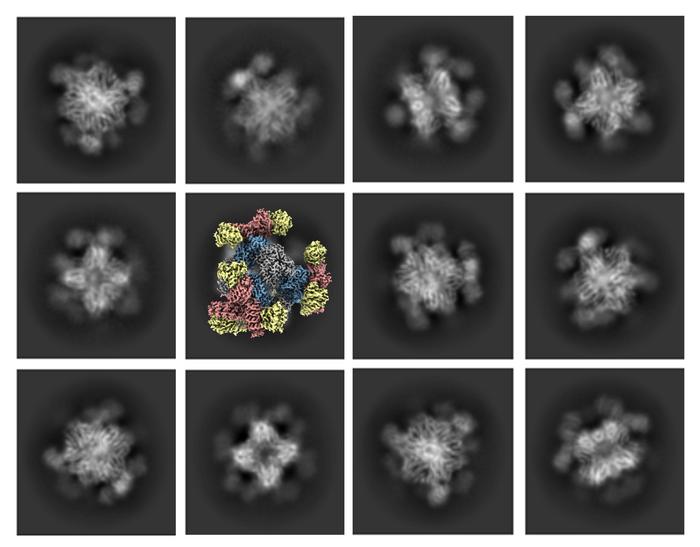
Now, UCLA biochemists, working with pharmaceutical industry scientists, have developed a solution that will make it possible for cryo-EM to acquire high-quality images of smaller protein molecules, too. The scientists engineered a 20 nanometer, cube-shaped protein structure, called a scaffold, with rigid tripod-like protrusions that hold the small proteins in place.
The scaffold can be digitally removed from the picture when the imaging is being processed, leaving a composite 3D image of just the small protein scientists are analyzing.
Small and medium-sized proteins are a hot point for research on potential new drugs that might one day be used to fight some of the most intractable human illnesses. The advance, which was tested on a protein that scientists are studying for its possible use in cancer treatments, can be customized for almost any small protein. Researchers expect that expanding cryo-EM’s imaging capabilities will help them identify specific locations on proteins that they can target for therapeutic purposes.
A paper about the new research is published in Proceedings of the National Academy of Sciences.
In cryo-EM, scientists use a cryo-electron microscope to send a beam of electrons through frozen samples of material, leaving behind an image of the thousands of molecules — such as proteins — in the sample. The molecules are imaged exactly as they lie in the sample, producing thousands of 2D photographs of the molecule taken from different angles. Computer processing reconciles all of those photographs to formulate a correct 3D image — separating the background, grouping images with similar orientations together and generating a high-resolution, 3D image of a single molecule.
But when it comes to imaging the smallest protein molecules, their tiny size makes it impossible to ascertain their orientations in the images, which results in relatively low-resolution images.
In previous studies, scientists attempted to solve the problem by attaching small molecules to larger scaffolds, but those experiments demonstrated that if the small molecules were attached too flexibly, they would protrude from the scaffold at different angles and orientations — which would still produce blurry images.
“The images are blurry because the computer can’t create a distinct composite image when it can’t determine the orientations accurately,” said Todd Yeates, a UCLA distinguished professor emeritus of biochemistry, interim director of the UCLA–Department of Energy Institute for Genomics and Proteomics and the paper’s corresponding author.
In the new study, the scaffold created by the scientists has tripod-shaped protrusions that capture the proteins and hold them firmly in place, which yielded the higher-resolution images they were aiming for.
“Attaching the small molecules rigidly to larger scaffolds creates particles that are large enough to be imaged, and which all have precisely the same 3D shape,” Yeates said. “And from there, the process works as usual to construct the high-resolution 3D image.”
Roger Castells-Graells, a UCLA postdoctoral researcher and the study’s lead author, said the scientists first tried another shape for the scaffold before landing on the version with tripod-shaped protrusions.
“At first we used one ‘stick’ pointing outward and that didn’t work as well,” he said. “The new scaffold has protrusions that point toward each other in triplets — like tripods — that hold the protein firmly.”
The researchers tested their scaffold by attempting to create images of a protein called KRAS. KRAS encourages cells to proliferate and is involved in about 25% of human cancers; it’s of particular interest to pharmaceutical researchers because identifying specific locations on the protein that are related to its cancer-causing abilities could help scientists design drugs that neutralize activity at those locations — which could be one path toward treating cancer.
Using cryo-EM and the scaffold they developed, the UCLA-led team was able to observe the atomic structure of KRAS attached to a drug molecule that is being studied as part of a potential treatment for lung cancer. Their work proved that the new scaffolded cryo-EM approach can illuminate how drug molecules bind with and inhibit cellular proteins like KRAS, and could help guide the development of more effective drugs.
The potential applications for the new advance don’t stop with cancer drugs, Castells-Graells said.
“Our scaffold is modular and can be assembled in any configuration to capture and hold all kinds of small protein molecules,” he said.
The research was supported by the National Institutes of Health and was a collaboration with scientists from Astra-Zeneca, whose team was led by Chris Phillips, and Gandeeva Therapeutics, whose team was led by Sriram Subramaniam.
UCLA has filed a patent on the new technology, and Yeates, Castells-Graells and colleagues have started a new company, AvimerBio, to help develop new commercial applications using the new methods, in collaboration with leading pharmaceutical companies.
Journal
Proceedings of the National Academy of Sciences



