Reston, VA—A new imaging agent, 68Ga-ABY-025, can predict early metabolic response to human epidermal growth factor receptor 2 (HER2)-targeted treatment in HER2-positive metastatic breast cancer patients, according to new research published in the September issue of The Journal of Nuclear Medicine. By providing whole-body quantification of HER2 expression, 68Ga-ABY-025 PET/CT can play a valuable role in treatment planning and could spare patients from unnecessary drug-related side effects.
Credit: Images created by Alhuseinalkhudhur et al., Uppsala University Hospital in Uppsala, Sweden.
Reston, VA—A new imaging agent, 68Ga-ABY-025, can predict early metabolic response to human epidermal growth factor receptor 2 (HER2)-targeted treatment in HER2-positive metastatic breast cancer patients, according to new research published in the September issue of The Journal of Nuclear Medicine. By providing whole-body quantification of HER2 expression, 68Ga-ABY-025 PET/CT can play a valuable role in treatment planning and could spare patients from unnecessary drug-related side effects.
Up to 20 percent of breast cancer patients have HER2 overexpression, making HER2 an attractive therapy target for both new and recurring cases. However, since breast cancer is a heterogeneous disease and HER2 expression may vary within the same patient and over time, HER2-targeted treatment failure is common, and cure remains rare in metastatic disease.
“While HER2 status can be confirmed by biopsy results in early-stage breast cancer, it is more complicated in advanced disease where multiple metastases might have a heterogeneous HER2 expression,” said Ali Alhuseinalkhudhur, MD, PhD candidate in the Department of Immunology, Genetics, and Pathology at Uppsala University in Uppsala, Sweden. “Imaging the entire body rather than just the primary cancer site can give physicians a total estimate of HER2 expression so they can plan targeted therapies appropriately.”
Researchers investigated 68Ga-ABY-025 PET as a noninvasive tool for whole-body HER2-receptor quantification. Forty patients with known positive HER2 status were included in the study: 19 with primary breast cancer and 21 with metastatic breast cancer (median of three previous treatments).
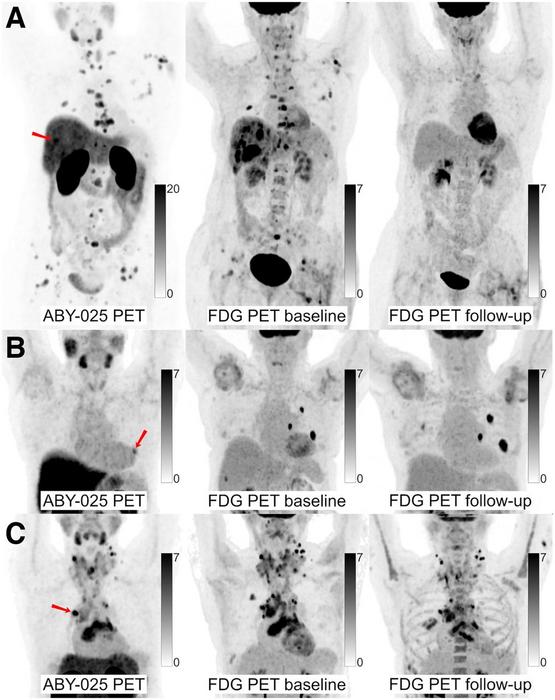
68Ga-ABY-025 PET/CT, 18F-FDG PET/CT, and core-needle biopsies from targeted lesions were performed at the baseline for each patient. 18F-FDG PET/CT was repeated after two cycles of therapy to calculate the directional change in tumor lesion glycolysis. Tracer uptake was measured in up to five of the largest lesions per patient, including the biopsied lesion. Standardized uptake values from 68Ga-ABY-025 PET/CT were then compared with the biopsied HER2 status and change in tumor lesion glycolysis.
68Ga-ABY-025 PET/CT successfully enabled quantification of HER2 expression, and uptake correlated significantly with metabolic response among patients, particularly in those with metastatic breast cancer. Also, an inverse association was found between the number of previous treatments and the metabolic response to the current treatment; the more treatments received, the higher the 68Ga-ABY-025 required to induce a metabolic response.
“The ability of 68Ga-ABY-025 PET/CT to provide a whole-body visualization of HER2 expression and to predict metabolic response is advantageous and exceeded the biopsy-based approach for metastatic breast cancer patients,” noted Alhuseinalkhudhur. “HER2-based imaging tools might provide a solution in situations where biopsies cannot be performed safely or when biopsy results are inconsistent. In addition, a PET-based approach to evaluate the appropriateness of targeted therapy might help avoid unnecessary side effects and might provide a more personalized opportunity for timely therapy corrections.”
He continued, “Our work is a strong example of the development toward personalized medicine. We hope that the value of quantification of biomarkers such as HER2 expression in cancer patients will be appreciated to a larger extent and introduced into nuclear medicine practice for both diagnostic imaging and therapy.”
This study was made available online in July 2023.
The authors of “Human Epidermal Growth Factor Receptor 2–Targeting [68Ga]Ga-ABY-025 PET/CT Predicts Early Metabolic Response in Metastatic Breast Cancer” include Ali Alhuseinalkhudhur, Division of Nuclear Medicine and PET, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden, and Department of Immunology, Genetics, and Pathology, Uppsala University, Uppsala, Sweden; Henrik Lindman and Vladimir Tolmachev, Department of Immunology, Genetics, and Pathology, Uppsala University, Uppsala, Sweden; Per Liss, Division of Radiology, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden; Tora Sundin, Clinical Research and Development Unit, Uppsala University Hospital, Uppsala, Sweden; Fredrik Y. Frejd, Department of Immunology, Genetics, and Pathology, Uppsala University, Uppsala, Sweden, and Affibody AB, Solna, Sweden; Johan Hartman and Caroline Rönnlund, Department of Oncology–Pathology, Karolinska Institute, Stockholm, Sweden, and Department of Clinical Pathology and Cancer Diagnostics, Karolinska University Hospital, Stockholm, Sweden; Victor Iyer, Irina Velikyan, and Jens Sörensen, Division of Nuclear Medicine and PET, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden; Joachim Feldwisch, Affibody AB, Solna, Sweden; and Mark Lubberink, Division of Nuclear Medicine and PET, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden, and Department of Medical Physics, Uppsala University Hospital, Uppsala, Sweden.
Visit the JNM website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed or follow us on LinkedIn.
###
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected].
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed 15 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit www.snmmi.org.
Journal
Journal of Nuclear Medicine
DOI
10.2967/jnumed.122.265364
Article Title
Human Epidermal Growth Factor Receptor 2–Targeting [68Ga]Ga-ABY-025 PET/CT Predicts Early Metabolic Response in Metastatic Breast Cancer
Article Publication Date
1-Sep-2023
COI Statement
This work was partially supported by grants from the Swedish Breast Cancer Association, Swedish Cancer Foundation (19 0507 Pj), Roche AB Sweden, and the Percy Falk Foundation. Fredrik Frejd and Joachim Feldwisch are employees and own shares in Affibody AB. Jens Sörensen received clinical advisor remunerations from Affibody AB. Johan Hartman obtained speaker’s honoraria or advisory board remunerations from Roche, Novartis, Pfizer, Eli Lilly, MSD, Veracyte, and ExactSciences and received institutional research support from Cepheid, Roche, and Novartis.