Pancreatic cancer is a complex and deadly solid tumor that poses significant challenges for both diagnosis and treatment. It is known for its aggressive local invasion and tendency for distant metastases, resulting in around 227,000 fatalities worldwide each year. Given the significant challenges posed by pancreatic cancer, extensive efforts have been undertaken to develop innovative therapeutic strategies that can improve treatment outcomes for this disease. To provide a potential approach, the research groups of Weihong Tan and Xue-Qiang Wang at the Hangzhou Institute of Medicine used phosphorothioate backbone modification to enhance the recognition ability, cellular uptake capacity, and biostability of an aptamer that targets pancreatic cancer cells. This modification led to the efficient and safer therapeutic agent delivery to pancreatic cancer.
Credit: ©Science China Press
Pancreatic cancer is a complex and deadly solid tumor that poses significant challenges for both diagnosis and treatment. It is known for its aggressive local invasion and tendency for distant metastases, resulting in around 227,000 fatalities worldwide each year. Given the significant challenges posed by pancreatic cancer, extensive efforts have been undertaken to develop innovative therapeutic strategies that can improve treatment outcomes for this disease. To provide a potential approach, the research groups of Weihong Tan and Xue-Qiang Wang at the Hangzhou Institute of Medicine used phosphorothioate backbone modification to enhance the recognition ability, cellular uptake capacity, and biostability of an aptamer that targets pancreatic cancer cells. This modification led to the efficient and safer therapeutic agent delivery to pancreatic cancer.
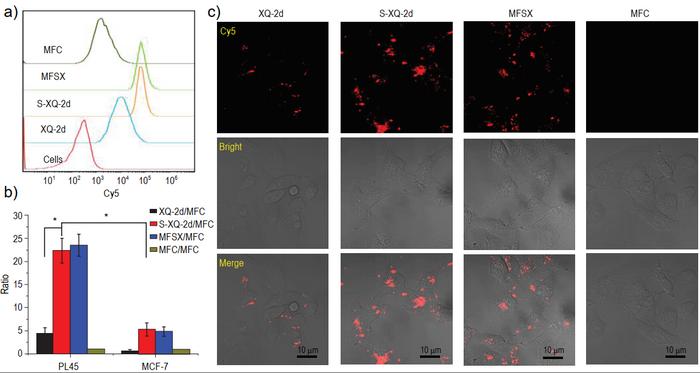
The study found that total phosphorothioate backbone modification significantly improved the biological stability of the aptamer in serum. Moreover, the researchers successfully prepared an aptamer-drug conjugate by coupling the aptamer with mitomycin C, a potent anti-tumor drug. Compared to the unmodified aptamer-drug conjugate, the modified aptamer-drug conjugate exhibited significantly improved binding and endocytosis ability towards targeted pancreatic cancer cells. Additionally, its toxicity towards non-targeted cells was significantly reduced, and it exhibited a longer blood circulation half-life in rats. Mouse imaging experiments also showed that the modified conjugate had improved infiltration ability to tumor sites and significantly prolonged retention time compared to the unmodified conjugate. These findings highlight the potential of total thio-modification as a means of regulating the biomedical properties of aptamers and their related therapeutic reagents, paving the way for the clinical translation of aptamers.
###
See the article:
Regulating the properties of XQ-2d for targeted therapeutic agents delivery to pancreatic cancers
https://doi.org/10.1093/nsr/nwad113
Journal
National Science Review
DOI
10.1093/nsr/nwad113



