Eczema is an inflammatory skin condition that often affects infants as young as one to two months. Among the various types of eczema seen in infants, early-onset atopic dermatitis (AD), characterized by psychological stress and sleep disorders, is particularly concerning. Studies have, in fact, identified that if left untreated, AD can increase the risk of allergic diseases such as food allergies and asthma—a progression also known as the “atopic march”. Early diagnosis and intervention of early-onset AD is needed to ensure the infant’s psychological and physical health.
Credit: Kao Corporation
Eczema is an inflammatory skin condition that often affects infants as young as one to two months. Among the various types of eczema seen in infants, early-onset atopic dermatitis (AD), characterized by psychological stress and sleep disorders, is particularly concerning. Studies have, in fact, identified that if left untreated, AD can increase the risk of allergic diseases such as food allergies and asthma—a progression also known as the “atopic march”. Early diagnosis and intervention of early-onset AD is needed to ensure the infant’s psychological and physical health.
However, it can be difficult to make a diagnosis of AD in infants as young as one or two months. Besides parents’ reluctance to seek medical advice and the infant’s inability to express their symptoms, the diagnosis of AD may be influenced by the doctor’s subjectivity and experience. Moreover, the use of accurate yet invasive diagnostic procedures for AD, such as skin biopsies, is difficult in infants. There is, thus, a need for new methods of diagnosing AD that are objective and non-invasive.
In an earlier study led by Project Leader Takayoshi Inoue from the Biological Science Research division at Kao Corporation, some of these researchers had identified that sebum contains measurable levels of human mRNA molecules. They hypothesized that analyzing the genetic expression of such RNA-containing sebum samples could reveal the molecular features of AD, and its underlying pathogenesis. Based on this discovery, the researchers developed a novel analytical method called “RNA monitoring” that enables human skin transcriptome analysis of the mRNA in sebum (skin surface lipids) collected from the skin using a simple oil-blotting film.
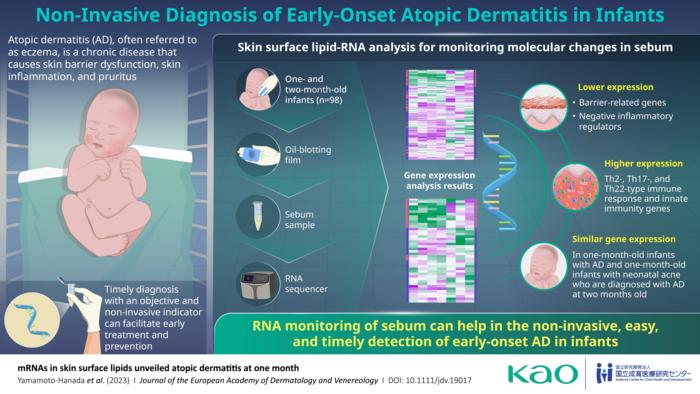
In this study published in the Journal of the European Academy of Dermatology and Venereology on March 10, 2023, the researchers verified the usefulness of this novel RNA monitoring method. This study was conducted in collaboration with Kiwako Yamamoto-Hanada and Yukihiro Ohya of the National Center for Allergy Research at the National Center for Child Health and Development, Japan. The main objective of this study was to determine if sebum RNA could provide reliable biomarkers for the detection of early-onset AD in infants.
The study population comprised a prospective cohort of 98 one- and two-month-old infants. In some of these infants, a diagnosis of AD was made according to the United Kingdom Working Party’s criteria. The researchers first collected sebum from the facial skin of all participating infants using a single oil-blotting film, in a non-invasive and easy procedure. Next, mRNA in skin surface lipids (SSLs) were extracted for performing transcriptome analysis, and lastly, subjected to data analysis for identifying their underlying molecular features of early-onset AD in infants.
The analysis revealed several genes with different expression between infants with and without AD. Specifically, the researchers observed that one-month-old infants with AD had lower expression of genes related to lipid metabolism and synthesis, tight junctions, antimicrobial peptides, and keratinization, and higher expression of genes related to Th2-, Th17- and Th22-type immune responses. These molecular changes in barrier function and inflammatory markers characterizing AD were not reported in earlier literature, especially in one-month old infants, largely owing to the invasiveness of common diagnostic procedures.
Most importantly, the team observed that via changes in the levels of these markers, sebum RNA could be used to detect the onset of AD well in advance. Explains Dr. Yamamoto-Hanada, Chief of the Allergy Center, “Our results confirm that the RNA monitoring method is useful for the early detection of AD in infants and may also be used for their treatment monitoring in the future.”
Hopefully, the availability of this simple, objective, and non-invasive diagnostic option for AD will encourage parents of infants with AD to opt for early consultation and therapeutical intervention of the condition. “Infants often have multiple eczemas and experience repeated exacerbations and remissions. With our method, the timely treatment of early-onset AD can be realized, enabling an improvement in the quality of life for infants with AD and their families,” says Dr. Yamamoto-Hanada.
We can only hope that the results of this research lessen the suffering of infants and their families and bring our society one step closer to being allergy-free!
About Kao
Kao creates high-value-added products and services that provide care and enrichment for the life of all people and the planet. Through its portfolio of over 20 leading brands such as Attack, Bioré, Goldwell, Jergens, John Frieda, Kanebo, Laurier, Merries, and Molton Brown, Kao is part of the everyday lives of people in Asia, Oceania, North America, and Europe. Combined with its chemical business, which contributes to a wide range of industries, Kao generates about 1,550 billion yen in annual sales. Kao employs about 35,400 people worldwide and has 136 years of history in innovation. Please visit the Kao Group website for updated information.
About the Allergy Center at the National Center for Child Health and Development
The Allergy Center at the National Center for Child Health and Development was established in June 2018. As the national core hospital for allergic diseases, the Allergy Center provides high quality clinical management of allergic disorders and promotes clinical research in allergology, operating through the Division of General Allergy, Division of Dermatological Allergy, and the Division of Gastrointestinal Allergy. It provides personalized treatment based on clinical guidelines and highly reliable medical evidence on areas such as food allergies, atopic dermatitis, bronchial asthma and associated diseases, and allergic rhinitis, to name a few.
Journal
Journal of the European Academy of Dermatology and Venereology
DOI
10.1111/jdv.19017
Method of Research
Experimental study
Subject of Research
Human tissue samples
Article Title
mRNAs in skin surface lipids unveiled atopic dermatitis at 1 month
Article Publication Date
10-Mar-2023
COI Statement
Kiwako Yamamoto-Hanada received JSPS KAKENHI (Grant Number: 22K10545, 20H05678, 19H03569, 18H03101 and 17K13214), National Center for Child Health and Development (Grant Number: 2019B-1 and 2021B-12), Japanese Society of Allergology, Environmental Restoration and Conservation Agency, National Science, and Takano Medical, consulting fees from Abbvie, KAO, and bcase, and lecture fees from Abbvie, KAO, Torii Pharmaceutical, Maruho, Pfizer and Otsuka Pharmaceutical out of submitted work. She was a member of the Committee for Clinical Practice Guidelines for the Management of AD 2021 (the Japanese Society of Allergology, the Japanese Dermatology Association) out of submitted work. Mayako Saito-Abe received a grant from JSPS KAKENHI (Grant Number 22K17791) out of submitted work and a lecture fee from Sato Pharmaceutical out of submitted work. Kyoko Shima is an employee of KAO and has patent pending for a method of detecting AD (2020JP-081503, PCT/JP2021/017112, 2021JP-151505, PCT/JP2021/034174). Satoko Fukagawa is an employee of KAO and has patent pending for a method of detecting AD (PCT/JP2021/017112). Yuya Uehara is an employee of KAO and has patent pending for a method of detecting AD (2021JP-151505, PCT/JP2021/034174). Yui Ueda is an employee of KAO. Maeko Iwamura is an employee of KAO. Takatoshi Murase is an employee of KAO. Tetsuya Kuwano is an employee of KAO and has patent pending for a method of detecting AD (PCT/JP2021/017112). Takayoshi Inoue is an employee of KAO and has patent pending for a method of detecting AD (2020JP-081503, PCT/JP2021/017112, 2021JP-151505, PCT/JP2021/034174). Yukihiro Ohya received the joint research grants and consulting fees from KAO related to this study. He obtained grants from Japan Agency for Medical Research and Development (Grant Number: JP18ek0410027h0003, JP20ek0410054h0002, JP21ek0410067h0002 and JP19ek0410037h0003), Health Labour Sciences Research Grant (Grant Number: 202111023A, 201913001B, 201911035B and 201913007B), JSPS KAKENHI (Grant Number: 18H03101, 19H03569 and 20H01622), National Center for Child Health and Development (2019E-1), Alcare, Fam’s Baby, Yakult, Duskin, Maruho, Novartis, Torii Pharmaceutical and Natural Science, and consulting fees from Abbvie, bcase, Kao, Maruho, Otsuka pharmaceutical, Sanofi/Regeneron, Torii/Japan Tabacco and Pfizer out of submitted work. He received lecture fees from Abbvie, Eli Lilly, Kyorin, Maruho, Mitsubishi Tanabe Pharma, Pola Pharma, Sanofi, Shino-test, Sysmex, Shiseido, Thermo Fisher Diagnostic, Torii Pharmaceutical, Towa Pharmaceutical, Otsuka Pharmaceutical and Pfizer out of submitted work. He participated on the advisory board of Abbvie and Sanofi out of submitted work. He was the vice-chairman of the Committee for Clinical Practice Guidelines for the Management of AD 2021 (the Japanese Society of Allergology) out of submitted work. He is a board member of the Japanese Society of Allergology and Japanese Society of Behavioral Medicine, the vice-chairman of the Japanese Society of Pediatric Dermatology out of submitted work.




