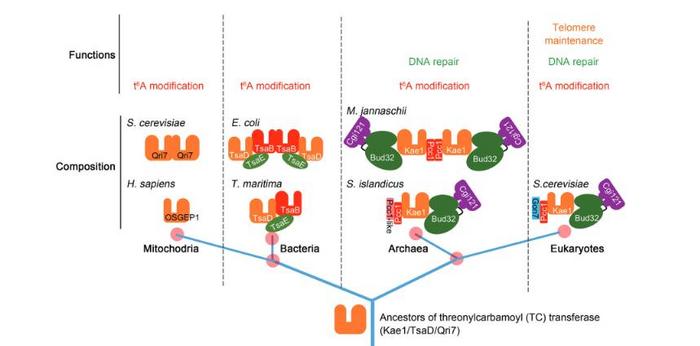
KEOPS is composed of Pcc1, Kae1, Bud32, Cgi121, and the eukaryotes-specific fifth subunit Gon7, and functions in N6-threonylcarbamoyl adenosine (t6A) modification of tRNAs, DNA repair, and telomere maintenance, etc. However, the origin of eukaryotic Gon7 subunit and whether archaeal KEOPS functions beyond t6A modification is unclear. Firstly, the researchers found a protein annotated as Pcc1-like co-purified with Pcc1 and Kae1 in the hyperthermophilic archaeon Sulfolobus islandicus REY15A. Interestingly, Pcc1-like is distributed in most archaea, which is possibly a counterpart of eukaryotes Gon7. To verify this hypothesis, they constructed the Pcc1-Kae1 subcomplex in different conditions. The results showed that Pcc1-like disrupts the dimerization of the Kae1:Pcc1 subcomplex, just like the function of Gon7 in eukaryotes. Structure modeling also supports this hypothesis. “Archaea Pcc1-like is the putative functional and structural homolog of Gon7,” the first author Pengju says.
Credit: Beijing Zhongke Journal Publising Co. Ltd.
KEOPS is composed of Pcc1, Kae1, Bud32, Cgi121, and the eukaryotes-specific fifth subunit Gon7, and functions in N6-threonylcarbamoyl adenosine (t6A) modification of tRNAs, DNA repair, and telomere maintenance, etc. However, the origin of eukaryotic Gon7 subunit and whether archaeal KEOPS functions beyond t6A modification is unclear. Firstly, the researchers found a protein annotated as Pcc1-like co-purified with Pcc1 and Kae1 in the hyperthermophilic archaeon Sulfolobus islandicus REY15A. Interestingly, Pcc1-like is distributed in most archaea, which is possibly a counterpart of eukaryotes Gon7. To verify this hypothesis, they constructed the Pcc1-Kae1 subcomplex in different conditions. The results showed that Pcc1-like disrupts the dimerization of the Kae1:Pcc1 subcomplex, just like the function of Gon7 in eukaryotes. Structure modeling also supports this hypothesis. “Archaea Pcc1-like is the putative functional and structural homolog of Gon7,” the first author Pengju says.
By introducing a t6A modification system from the thermophilic bacteria Thermotoga miritima into S. islandicus REY15A, the researchers found that it can complement the t6A modification function but not other fundemental functions of the archaeal KEOPS. Combined with the studies of threonylcarbamoyl adenosine (TC) transferases in other organisms’ t6A modification, a model for the evolution of the KEOPS and other t6A modification systems was proposed. “All t6A modification systems may have common ancestors,” the author explained, “and eukaryotic KEOPS shares additional similarity in composition, structure and functions to archaeal KEOPS, which provide the evidence for the the archaeal origin of Eukaryotes theory”.
See the article:
The archaeal KEOPS complex possesses a functional Gon7 homolog and has an essential function independent of the cellular t6A modification level
https://doi.org/10.1002/mlf2.12051
Journal
mLife
DOI
10.1002/mlf2.12051
Article Title
The archaeal KEOPS complex possesses a functional Gon7 homolog and has an essential function independent of the cellular t6A modification level
Article Publication Date
8-Jan-2023




