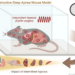
Research by scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) has demonstrated that the on-target molecular and cellular effects of medicines used to modulate the formation of new blood vessels (angiogenesis) in cardiovascular disorders and cancer are not responsible for the toxicity and vascular pathology triggered by these drugs. The study is published in Nature Cardiovascular Research.

Credit: CNIC
Research by scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) has demonstrated that the on-target molecular and cellular effects of medicines used to modulate the formation of new blood vessels (angiogenesis) in cardiovascular disorders and cancer are not responsible for the toxicity and vascular pathology triggered by these drugs. The study is published in Nature Cardiovascular Research.
Study leader Rui Benedito commented that “our results not only significantly increase our understanding of the biology of blood vessels, but will also help in the selection of the most effective and safe way to modulate angiogenesis in ischemic tissues or in cancer.”
The vascular system supplies oxygen and nutrients to the tissues and organs of the body. But the blood vessels do more than just conduct blood; they contribute actively to the physiology and homeostasis of all tissues and organs throughout life. Most blood vessels in the body are in an inactive state, which they maintain by expressing a large number of genes, including the genes of the signaling pathway mediated by Delta ligands and Notch receptors.
Several drugs have been developed in recent years that either block or induce angiogenesis in cardiovascular disorders or cancer.
A group of these compounds in clinical use inhibit different components of the Delta-Notch signaling pathway, which plays important roles in angiogenesis and in the maintenance of blood vessels in the inactive state. These compounds, by modulating the growth of blood vessels, efficiently block tumor growth. These compounds are also able to induce angiogenesis in ischemic tissues, thereby improving tissue regeneration and function.
However, these drugs can also cause vascular injury in organs with no previous disease, including the liver and heart, and this has reduced clinical interest in their use.
Until now, this vascular toxicity was thought to be due to the expression of genes that promote angiogenesis, leading the the appearance of neoplasms or tumors in the affected blood vessels.
Thanks to the use of advanced genetic mouse models, high-resolution confocal microscopy, and single-cell sequencing and proteomics techniques, the team discovered that the vascular toxicity linked to these drugs is instead due to a change in the vascular architecture that impedes correct blood flow.
“Our study shows that the vascular pathology that can result from treatment with these drugs is unrelated to the expression of genes involved in angiogenesis or the appearance of neoplasms” said Rui Benedito.
The researchers showed that these changes happen even when they blocked cell activation and the expression of angiogenesis-related genes.
Therefore, explained Rui Benedito, “although the neoplasms and the expression of genes associated with angiogenesis are associated with the change in vascular architecture, they are not the cause of this change.”
First autor Macarena Fernández Chacón explained that “after analyzing different genes and drugs targeting blood vessels, we have found new ways to control pathological angiogenesis without significantly affecting vascular architecture in other organs, thus avoiding toxicity.”
The study was supported by the European Research Council (ERC) through Starting Grant AngioGenesHD and Consolidator Grant AngioUnrestUHD, the CNIC Severo Ochoa Intramural Program, the Spanish Ministry of Science and Innovation, and Fundación “la Caixa”.
Journal
Nature Cardiovascular Research
DOI
10.1038/s44161-023-00272-4
Method of Research
Experimental study
Subject of Research
Cells
Article Title
‘Incongruence between transcriptional and vascular pathophysiological cell states’
Article Publication Date
29-May-2023