Fukuoka, Japan—A team of researchers have discovered that a mutation in a ribosomal protein found specifically in heart and skeletal muscle leads to impaired cardiac contractility in mice.
Credit: Kyushu University/Nakayama Lab
Fukuoka, Japan—A team of researchers have discovered that a mutation in a ribosomal protein found specifically in heart and skeletal muscle leads to impaired cardiac contractility in mice.
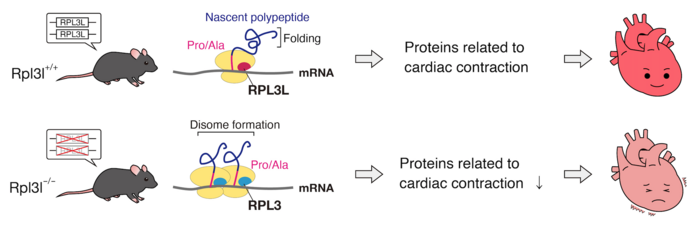
The mutation was found to delay the rate of translating mRNA, leading to ribosomes colliding and causing protein folding abnormalities. The abnormal proteins would then be targeted and degraded by the cell’s quality control system. Moreover, while the deficiency in the ribosomal protein, known as RPL3L, altered translation dynamics for the entire tissue, its effects were most pronounced for proteins related cardiac muscle contraction.
The study, published in Nature Communications, shines new insight into the dynamics of a molecule as fundamental as ribosomes. Furthermore, since RPL3L gene deficiencies have been found in humans with cardiomyopathy and atrial fibrillation, the team hopes their new findings can lead to future treatments.
You are likely familiar with the process of how cells produce the proteins and molecules that make the body function. DNA is transcribed into messenger RNA, or mRNA, which is then used as a blueprint to link amino acids together and build a protein. At the heart of the protein building process is the ribosome which reads the mRNA and translates that code into proteins.
Because of its fundamental function, ribosomes are found within all cells and were thought to be generally the same. However, recent studies have revealed the existence of differences in ribosomal structures.
“These differences in a ribosomal structure have shown to lead to translation specificity. For example, some ribosomes are better at producing proteins that control metabolism, or the cell cycle. It’s a new concept called Ribosome Heterogeneity,” explains Keiichi I. Nakayama of Kyushu University’s Medical Institute of Bioregulation who led the study. “We hypothesized that this heterogeneity exists between tissues. After screening for tissue-specific ribosomal proteins we found one that was only expressed in heart and skeletal muscle: RPL3L.”
To elucidate the function of RPL3L, the team studied the hearts of mice with a mutated RPL3L gene. As expected, echocardiographic analysis showed that they had reduced cardiac contractility. Their next step was to study why exactly this mutation led to such a condition. As it turns out, the RPL3L mutation was causing a ‘translational traffic jam’ for proteins critical in proper heart function.
“We found that the mutant RPL3L would delay translation for the proline and alanine codons on mRNA. This delay caused ribosomes to collide, resulting in proteins not folding correctly,” continues Nakayama. “Misfolded proteins would then be cleared out from the cell by its quality control system. More importantly, much of the misfolded proteins were ones involved in cardiac contraction.”
The team hopes that by deepening our understanding of the translation dynamics of ribosome such as RPL3L, they can better understand how its genetic mutations—found in patients with dilated cardiomyopathy and atrial fibrillation—can lead to heart disease.
“We are developing new understandings in the field of biology and medicine every day, even in something as fundamental as ribosomes. I’m exciting to see what we’ll find next,” concludes Nakayama.
###
For more information about this research, see “RPL3L-containing ribosomes determine translation elongation dynamics required for cardiac function,” Chisa Shiraishi, Akinobu Matsumoto, Kazuya Ichihara, Taishi Yamamoto, Takeshi Yokoyama, Taisuke Mizoo, Atsushi Hatano, Masaki Matsumoto, Yoshikazu Tanaka, Eriko Matsuura-Suzuki, Shintaro Iwasaki, Shouji Matsushima, Hiroyuki Tsutsui, Keiichi I. Nakayama Nature Communications, https://doi.org/10.1038/s41467-023-37838-6
About Kyushu University
Kyushu University is one of Japan’s leading research-oriented institutes of higher education since its founding in 1911. Home to around 19,000 students and 8,000 faculty and staff, Kyushu U’s world-class research centers cover a wide range of study areas and research fields, from the humanities and arts to engineering and medical sciences. Its multiple campuses—including one of the largest in Japan—are located around Fukuoka City, a coastal metropolis on the southwestern Japanese island of Kyushu that is frequently ranked among the world’s most livable cities and historically known as Japan’s gateway to Asia. Through its Vision 2030, Kyushu U will ‘Drive Social Change with Integrative Knowledge.’ Its synergistic application of knowledge will encompass all of academia and solve issues in society while innovating new systems for a better future.
Journal
Nature Communications
DOI
10.1038/s41467-023-37838-6
Method of Research
Experimental study
Subject of Research
Animals
Article Title
RPL3L-containing ribosomes determine translation elongation dynamics required for cardiac function
Article Publication Date
20-Apr-2023




