Researchers from the Department of Clinical Oncology, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong (HKUMed) discovered a novel subtype of Epstein-Barr Virus (EBV)-positive nasopharyngeal carcinoma (NPC) and EBV-associated immunosuppression in the tumour-microenvironment (TME). These findings have provided novel insights into the traditional NPC pathogenesis model and highlights EBV-specific communications in the TME as potential therapeutic target in NPC. The research has been published in eBioMedicine [link to publication].
Credit: The University of Hong Kong
Researchers from the Department of Clinical Oncology, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong (HKUMed) discovered a novel subtype of Epstein-Barr Virus (EBV)-positive nasopharyngeal carcinoma (NPC) and EBV-associated immunosuppression in the tumour-microenvironment (TME). These findings have provided novel insights into the traditional NPC pathogenesis model and highlights EBV-specific communications in the TME as potential therapeutic target in NPC. The research has been published in eBioMedicine [link to publication].
Background and Research Findings
NPC has a high incidence rate in Southeast Asia, in particular Guangdong and Hong Kong. Due to its worldwide rarity, studies on NPC heavily rely on local research teams, and its pathogenesis mechanism[1] remains largely unclear. In Hong Kong, NPC is the commonest cancer type for the men aged 20-44 and ranked 8th highest incidence rate among males.[2] Strikingly, EBV is detected in 95% of the Hong Kong cases. Having thorough understanding of NPC pathogenesis, in particular the role of EBV, is critical for advancing the clinical diagnosis and treatment for this deadly disease and is an active research topic in the field.
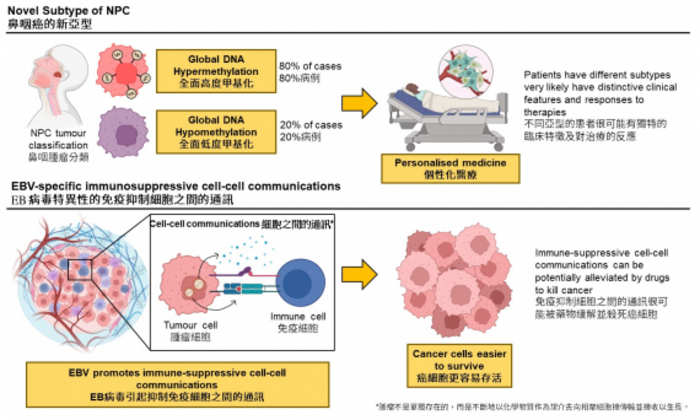
The research team used a cutting-edge bioinformatics approach to comprehensively decode the epigenetics[3] of the tumours dissected from NPC patients. EBV+NPC was believed to be massively dysregulated by the global DNA hypermethylation, a phenomenon that denotes a large-scale increase of methyl groups onto the DNA sequences within the cancer cells. These methyl-groups function like an ‘off-switch’ to inactivate tumour-suppressors that safeguard the cells from turning into a tumour, and thereby, promoting tumour development. Moreover, global DNA hypermethylation is rarely observed in non-EBV cancer types proposed to be associated with EBV and is a critical step in NPC pathogenesis.
The research team discovered that, in contrast to what was commonly believed, 20% of NPC cases were characterised by global DNA hypomethylation, which refers to a large-scale decrease of methyl groups onto the DNA sequences in the cancer cells. The study also discovered that EBV may reprogram the cell-cell communications[4] between the cancer cells and the immune cells, and consequently protect the cancer cells from being destroyed by the immune system.
Significance of the Study
‘Commonly infected by EBV, the NPC tumours carried distinctive methylation patterns. This finding is not well-recognised by the NPC development model. When global DNA hypomethylation occurs during NPC pathogenesis, whether it occurs as an alternative pathway in a subset of patients and its potential of predicting patients’ survival, clinical features, and response to therapies are critical for understanding NPC and providing personalized treatments for patients.’ commented Dr Dai Wei, Assistant Professor of the Department of Clinical Oncology, School of Clinical Medicine, HKUMed.
Professor Maria Li Lung, Emeritus Professor of the Department of Clinical Oncology, School of Clinical Medicine, HKUMed, added, ‘Since the immunosuppressive cell-cell communications were associated with EBV, these communications are highly-specific to the tumours and could be potential therapeutic targets and biomarkers in NPC.’ ‘We are now designing experiments to explore this feasibility and understand the clinical impacts of NPC subtypes. We hope the work can be beneficial to NPC patients in Hong Kong.’
About the research team
This research was co-supervised by Dr Dai Wei, Assistant Professor, and Professor Maria Li Lung, Emeritus Professor of the Department of Clinical Oncology, School of Clinical Medicine, HKUMed. Dr Larry Chow Ka-yue and Mr Dittman Chung Lai-shun from the Department of Clinical Oncology, School of Clinical Medicine, HKUMed, are the co-first authors, Dr Tao Lihua, Scientific Officer, provided support to the research.
The collaborators included Dr Chan Kui-fat and Dr Stewart Tung Yuk from Department of Clinical Oncology and Department of Clinical Pathology from the Tuen Mun Hospital, Hong Kong; Professor Roger Ngan Kai-cheong, Professor Ng Wai-tong, Professor Anne Lee Wing-mui, Professor Dora Kwong Lai-wan, Dr Victor Lee Ho-fun and Dr Lam Ka-on from the Department of the Clinical Oncology, School of Clinical Medicine, HKUMed; Dr Yau Chun-chung from Department of Oncology from Princess Margaret Hospital, Hong Kong; Professor Chen Honglin and Dr Liu Jiayan from Department of Microbiology, School of Biomedical Sciences, HKUMed.
About Department of Clinical Oncology
The Department of Clinical Oncology is an academic department specialized on a full spectrum of non-surgical cancer services including radiotherapy, chemotherapy, targeted therapy, and palliative care. The Department has two research laboratories including Laboratory of Cancer Molecular Genomics and Laboratory of Cancer Genetics and provides state-of-the-art cancer management at two hospitals (Queen Mary Hospital in Hong Kong and The University of Hong Kong-Shenzhen Hospital in China) in close collaboration with multiple disciplines.
Acknowledgement
This study was supported by the funding from Area of Excellence Scheme (AoE/M-06/08) and General Research Fund (17103218, 17102619, 17119618) by the Research Grants Council of the University Grants Committee and Seed Fund for Basic Research of The University of Hong Kong (201611159158). The team is also deeply grateful to Professor George Tsao Sai-wah in the Department of Anatomy, School of Biomedical Sciences, for generously sharing the essential nasopharyngeal cell line models and valuable comments on the work.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email ([email protected]).
[1] A pathogenesis model postulates the molecular mechanism of how a normal cell becomes a cancer cell, progresses into a bulk tumor, and metastasis.
[2] Hong Kong Cancer Registry, Hospital Authority. (2019). Overview of Hong Kong Cancer Statistics of 2019. Retrieved December 2, 2022, from https://www3.ha.org.hk/cancereg/; Hong Kong Cancer Registry, Hospital Authority. (2022). Top Ten Cancers. Retrieved November 28, 2022, from https://www3.ha.org.hk/cancereg/topten.html
[3] Epigenetics refers to the biochemical processes that control gene function, for instance, the expression of tumor-suppressor genes, without changing the DNA sequences. Epigenetic alterations are believed to be drivers of aging and various cancer types, including NPC.
[4] Rather than living alone, a tumor consistently receives/transmits messages in form of chemicals from/to the adjacent cells to grow and progress.
Journal
EBioMedicine
DOI
10.1016/j.ebiom.2022.104357
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Epigenomic landscape study reveals molecular subtypes and EBV-associated regulatory epigenome reprogramming in nasopharyngeal carcinoma
Article Publication Date
10-Nov-2022



