New York, NY—April 27, 2022—Engineered tissues have become a critical component for modeling diseases and testing the efficacy and safety of drugs in a human context. A major challenge for researchers has been how to model body functions and systemic diseases with multiple engineered tissues that can physiologically communicate – just like they do in the body. However, it is essential to provide each engineered tissue with its own environment so that the specific tissue phenotypes can be maintained for weeks to months, as required for biological and biomedical studies. Making the challenge even more complex is the necessity of linking the tissue modules together to facilitate their physiological communication, which is required for modeling conditions that involve more than one organ system, without sacrificing the individual engineered tissue environments.
Credit: Kacey Ronaldson-Bouchard/Columbia Engineering
New York, NY—April 27, 2022—Engineered tissues have become a critical component for modeling diseases and testing the efficacy and safety of drugs in a human context. A major challenge for researchers has been how to model body functions and systemic diseases with multiple engineered tissues that can physiologically communicate – just like they do in the body. However, it is essential to provide each engineered tissue with its own environment so that the specific tissue phenotypes can be maintained for weeks to months, as required for biological and biomedical studies. Making the challenge even more complex is the necessity of linking the tissue modules together to facilitate their physiological communication, which is required for modeling conditions that involve more than one organ system, without sacrificing the individual engineered tissue environments.
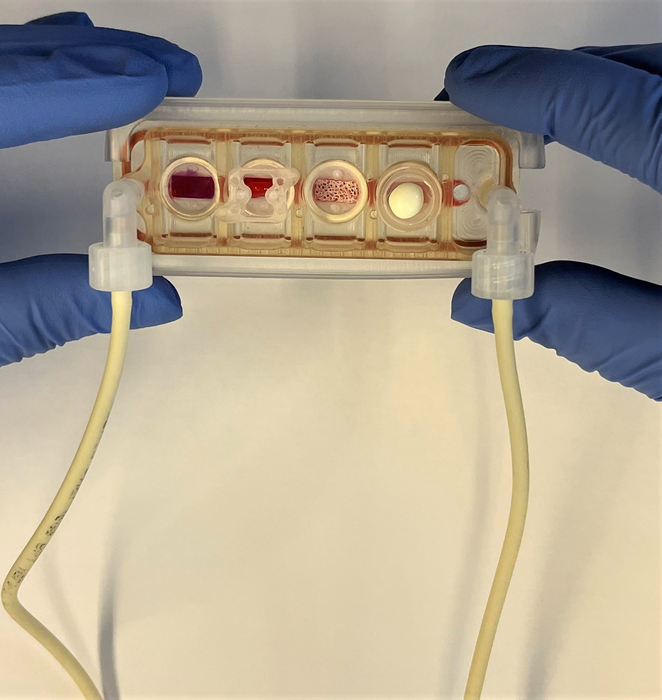
Novel plug-and-play multi-organ chip, customized to the patient
Up to now, no one has been able to meet both conditions. Today, a team of researchers from Columbia Engineering and Columbia University Irving Medical Center reports that they have developed a model of human physiology in the form of a multi-organ chip consisting of engineered human heart, bone, liver, and skin that are linked by vascular flow with circulating immune cells, to allow recapitulation of interdependent organ functions. The researchers have essentially created a plug-and-play multi-organ chip, which is the size of a microscope slide, that can be customized to the patient. Because disease progression and responses to treatment vary greatly from one person to another, such a chip will eventually enable personalized optimization of therapy for each patient. The study is the cover story of the April 2022 issue of Nature Biomedical Engineering.
“This is a huge achievement for us—we’ve spent ten years running hundreds of experiments, exploring innumerable great ideas, and building many prototypes, and now at last we’ve developed this platform that successfully captures the biology of organ interactions in the body,” said the project leader Gordana Vunjak-Novakovic, University Professor and the Mikati Foundation Professor of Biomedical Engineering, Medical Sciences, and Dental Medicine.
Inspired by the human body
Taking inspiration from how the human body works, the team has built a human tissue-chip system in which they linked matured heart, liver, bone, and skin tissue modules by recirculating vascular flow, allowing for interdependent organs to communicate just as they do in the human body. The researchers chose these tissues because they have distinctly different embryonic origins, structural and functional properties, and are adversely affected by cancer treatment drugs, presenting a rigorous test of the proposed approach.
“Providing communication between tissues while preserving their individual phenotypes has been a major challenge,” said Kacey Ronaldson-Bouchard, the study’s lead author and an associate research scientist in Vunjak-Novakovic’s Laboratory for Stem Cells and Tissue Engineering. “Because we focus on using patient-derived tissue models we must individually mature each tissue so that it functions in a way that mimics responses you would see in the patient, and we don’t want to sacrifice this advanced functionality when connecting multiple tissues. In the body, each organ maintains its own environment, while interacting with other organs by vascular flow carrying circulating cells and bioactive factors. So we chose to connect the tissues by vascular circulation, while preserving each individual tissue niche that is necessary to maintain its biological fidelity, mimicking the way that our organs are connected within the body. ”
Optimized tissue modules can be maintained for more than a month
The group created tissue modules, each within its optimized environment and separated them from the common vascular flow by a selectively permeable endothelial barrier. The individual tissue environments were able to communicate across the endothelial barriers and via vascular circulation. The researchers also introduced into the vascular circulation the monocytes giving rise to macrophages, because of their important roles in directing tissue responses to injury, disease, and therapeutic outcomes.
All tissues were derived from the same line of human induced pluripotent stem cells (iPSC), obtained from a small sample of blood, in order to demonstrate the ability for individualized, patient-specific studies. And, to prove the model can be used for long-term studies, the team maintained the tissues, which had already been grown and matured for four to six weeks, for an additional four weeks, after they were linked by vascular perfusion.
Using the model to study anticancer drugs
The researchers also wanted to demonstrate how the model could be used for studies of an important systemic condition in a human context and chose to examine the adverse effects of anticancer drugs. They investigated the effects of doxorubicin — a broadly used anticancer drug — on heart, liver, bone, skin, and vasculature. They showed that the measured effects recapitulated those reported from clinical studies of cancer therapy using the same drug.
The team developed in parallel a novel computational model of the multi-organ chip for mathematical simulations of drug’s absorption, distribution, metabolism, and secretion. This model correctly predicted doxorubicin’s metabolism into doxorubicinol and its diffusion into the chip. The combination of the multi-organ chip with computational methodology in future studies of pharmacokinetics and pharmacodynamics of other drugs provides an improved basis for preclinical to clinical extrapolation, with improvements in the drug development pipeline.
“While doing that, we were also able to identify some early molecular markers of cardiotoxicity, the main side-effect that limits the broad use of the drug. Most notably, the multi-organ chip predicted precisely the cardiotoxicity and cardiomyopathy that often require clinicians to decrease therapeutic dosages of doxorubicin or even to stop the therapy,” said Vunjak-Novakovic.
Collaborations across the university
The development of the multi-organ chip began from a platform with the heart, liver, and vasculature, nicknamed the HeLiVa platform. As is always the case with Vunjak-Novakovic’s biomedical research, collaborations were critical for completing the work. These include the collective talent of her laboratory, Andrea Califano and his systems biology team (Columbia University), Christopher S. Chen (Boston University) and Karen K. Hirschi (University of Virginia) with their expertise in vascular biology and engineering, Angela M. Christiano and her skin research team (Columbia University), Rajesh K. Soni of the Proteomics Core at Columbia University, and the computational modeling support of the team at CFD Research Corporation.
A multitude of applications, all in individualized patient-specific contexts
The research team is currently using variations of this chip to study, all in individualized patient-specific contexts: breast cancer metastasis; prostate cancer metastasis; leukemia; effects of radiation on human tissues; the effects of SARS-CoV-2 on heart, lung, and vasculature; the effects of ischemia on the heart and brain; and the safety and effectiveness of drugs. The group is also developing a user-friendly standardized chip for both academic and clinical laboratories, to help utilize its full potential for advancing biological and medical studies.
Vunjak-Novakovic added, “After ten years of research on organs-on-chips, we still find it amazing that we can model a patient’s physiology by connecting millimeter sized tissues — the beating heart muscle, the metabolizing liver, and the functioning skin and bone that are grown from the patient’s cells. We are excited about the potential of this approach. It’s uniquely designed for studies of systemic conditions associated with injury or disease, and will enable us to maintain the biological properties of engineered human tissues along with their communication. One patient at a time, from inflammation to cancer!”
###
About the Study
Journal: Nature Biomedical Engineering
The study is titled “A multi-organ chip with matured tissue niches linked by vascular flow”
Authors are: Kacey Ronaldson-Bouchard1, Diogo Teles1,2,3, Keith Yeager1, Daniel Naveed Tavakol 1, Yimu Zhao1, Alan Chramiec1, Somnath Tagore4, Max Summers1, Sophia Stylianos1, Manuel Tamargo1, Busub Marcus Lee1, Susan P. Halligan1, Erbil Hasan Abaci5, Zongyou Guo5, Joanna Jacków5, Alberto Pappalardo5, Jerry Shih6, Rajesh K. Soni7, Shivam Sonar8, Carrie German8, Angela M. Christiano5,9, Andrea Califano4,7,10-13, Karen K. Hirschi14, Christopher S. Chen6, Andrzej Przekwas8 and Gordana Vunjak-Novakovic1,12,15
1 Department of Biomedical Engineering, Columbia University, New York City, NY, USA.
2 Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal.
3 ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarāes, Braga, Portugal.
4 Department of Systems Biology, Columbia University, New York City, NY, USA.
5 Department of Dermatology, Columbia University, New York City, NY, USA.
6 Department of Biomedical Engineering, Boston University, The Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA, USA.
7 Herbert Irving Comprehensive Cancer Center, Columbia University, New York City, NY, USA.
8 CFD Research Corporation, Huntsville, AL, USA.
9 Department of Genetics and Development, Columbia University, New York City, NY, USA.
10 Department of Biomedical Informatics, Columbia University, New York, New York.
11 Department of Biochemistry and Molecular Biophysics, Columbia University, New York, New York.
12 Department of Medicine, Vagelos College of Physicians and Surgeons, Columbia University, New York City, NY, USA.
13 J.P. Sulzberger Columbia Genome Center, New York, NY, USA.
14 Department of Cell Biology, University of Virginia, Charlottesville, VA, USA.
15 College of Dental Medicine, Columbia University, New York, NY, USA.
The study was supported by the NIH (UG3 EB025765, P41 EB027062, and R01 CA249799 to G.V.-N.; R35 CA197745, S10 OD012351, and S10 OD021764 to An.C.; UL1 TR001873 to the Irving Institute for Clinical and Translational Research; P30 CA013696 to the Confocal and Specialized Microscopy Shared Resource), NSF (Engineering Research Center EEC-1647837 to C.S.C. and G.V.-N., Graduate Research Fellowship DGE1644869 to D.N.T.), and F.C.T. (PD/BD/105819/2014 to D.T.).
G.V.-N. is a co-founder and board director of epiBone, Tara Biosystems, Xylyx Bio, and Immplacate and holds equity in all four companies. K.R-B. and Y.Z. are co-founders of TARA Biosystems and hold equity in the company. S.P.H. holds equity in Xylyx Bio. An.C. is founder, equity holder, and consultant of DarwinHealth, Inc., a company that has licensed some of the algorithms used in this manuscript from Columbia University.
Columbia University is also an equity holder in DarwinHealth Inc. The other authors declare no competing interests.
Journal
Nature Biomedical Engineering
DOI
10.1038/s41551-022-00882-6
Article Title
A multi-organ chip with matured tissue niches linked by vascular flow
Article Publication Date
27-Apr-2022
COI Statement
G.V.-N. is a co-founder and board director of epiBone, Tara Biosystems, Xylyx Bio, and Immplacate and holds equity in all four companies. K.R-B. and Y.Z. are co-founders of TARA Biosystems and hold equity in the company. S.P.H. holds equity in Xylyx Bio. An.C. is founder, equity holder, and consultant of DarwinHealth, Inc., a company that has licensed some of the algorithms used in this manuscript from Columbia University.
Columbia University is also an equity holder in DarwinHealth Inc. The other authors declare no competing interests.



