Researchers at the Okinawa Institute of Science and Technology Graduate University (OIST) have revealed how poxviruses build their scaffold – a temporary protein coat that forms and disappears as the virus matures.
Reporting today in Nature Communications, the scientists revealed the structure of a protein called D13, in near-atomic resolution, and showed how it assembles with other copies of D13 to form scaffold-like structures.
“D13 is a key target for research, because if you know how the scaffold is assembled, you can design new drugs that prevent it from forming,” said Professor Jaekyung Hyun, a former staff scientist in the OIST Molecular Cryo-Electron Microscopy Unit, and now Assistant Professor at Pusan National University in South Korea. “If the scaffold can’t form, then replication of the virus stops.”
D13 is a trimer protein, as it is formed from three identical protein chains. Once synthesized, it acts as a scaffold building block for the Vaccinia virus – a harmless strain developed in the laboratory as a vaccine against smallpox. Researchers now use the Vaccinia virus as a model for all poxviruses.
“Smallpox is the most famous and lethal disease caused by a poxvirus, with 1 in 3 infected people dying,” said Professor Wolf, who leads the Molecular Cryo-Electron Microscopy Unit. “But while smallpox has been eradicated in the wild, there are fears that it could be used as a bioweapon. Also, numerous other poxviruses still infect humans and livestock, so further research into how these viruses replicate is essential.”
The scaffold seen in immature poxviruses is of particular interest to scientists, as the structure differs from the protein coats typically seen in viruses.
While most viruses have regular and symmetrical structures, poxvirus scaffolds have roughly spherical honeycomb lattices, which vary in shape between each viral particle.
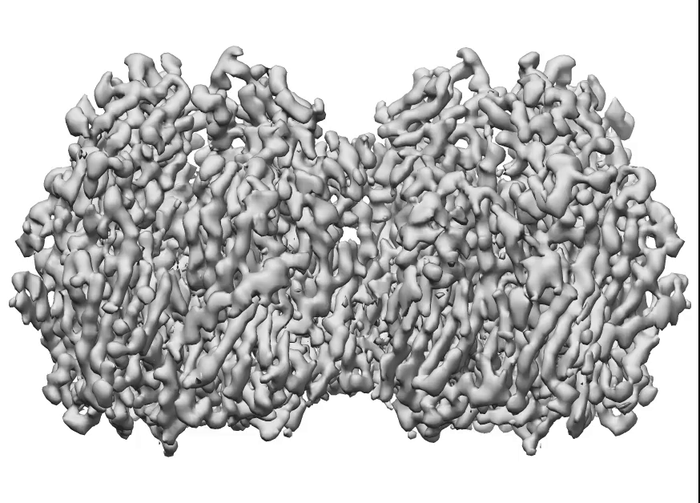
To determine how these blocks assemble into these spherical honeycomb lattices, the research team used cryo-electron microscopy (cryo-EM) – a technique in which samples are frozen in liquid nitrogen and probed by electrons – to generate 3D images of both single D13 protein trimers and two connected D13 protein trimers, at the highest resolution seen so far.
They found that the two proteins joined together with a slight twist between their trimer axes, creating a curve that is key for forming a spherical shape.
However, when the team used computer modelling to extend the interaction to multiple D13 proteins, they didn’t fit together properly.
“This told us that there must be at least one other way for the two proteins to interact, that we hadn’t yet seen,” said Prof. Hyun.
The researchers also found that when they compared the single D13 protein to the two D13 proteins joined together, a small helix structure at the end of the protein chains had shifted. Previously, the helix structure was buried deep into the pocket where the two proteins interact, suggesting that its repositioning was critical for the two proteins to connect.
To explore the role of the helix structure further, the research team made modified D13 proteins and then used cryo-electron tomography to look at how they self-assembled when placed in solution. When a purification tag was added to the helix, the proteins formed spherical structures similar to the immature poxvirus scaffold. And when the helix was completely removed, the researchers were surprised to see the formation of cylindrical tubes.
Using cryo-EM, the research team were able to capture high-res images of these cylinders and zoom in on the honeycomb structure. They identified a second way for the dimers to interact and found that when they modelled alternating patterns of interaction, the D13 proteins fit together to form the hexagonal honeycomb pattern.
Both modes of how the proteins interacted required the small helix structure to shift, with the proteins then held together by the attraction between positively and negatively charged amino acids.
Overall, the researchers proposed that displacement of the helix was essential for forming poxvirus scaffold, and likely acted as a trigger for the assembly to begin.
“When the poxvirus replicates inside cells, the scaffold forms in association with a lipid membrane,” explained Prof. Wolf. “The helix structure is hydrophobic, which means that it would shift towards to the water-free environment of the lipid membrane, freeing up the pocket where the D13 proteins interact.”
The discovery of the helix’s role in assembly could be a promising new avenue of research for antiviral drug discovery, emphasized Prof. Hyun.
“If we can design a drug that binds really strongly into the pocket where the helix usually sits, it would interfere with formation of the scaffold,” he said. “This is one of my next goals.”
Credit: OIST
Researchers at the Okinawa Institute of Science and Technology Graduate University (OIST) have revealed how poxviruses build their scaffold – a temporary protein coat that forms and disappears as the virus matures.
Reporting today in Nature Communications, the scientists revealed the structure of a protein called D13, in near-atomic resolution, and showed how it assembles with other copies of D13 to form scaffold-like structures.
“D13 is a key target for research, because if you know how the scaffold is assembled, you can design new drugs that prevent it from forming,” said Professor Jaekyung Hyun, a former staff scientist in the OIST Molecular Cryo-Electron Microscopy Unit, and now Assistant Professor at Pusan National University in South Korea. “If the scaffold can’t form, then replication of the virus stops.”
D13 is a trimer protein, as it is formed from three identical protein chains. Once synthesized, it acts as a scaffold building block for the Vaccinia virus – a harmless strain developed in the laboratory as a vaccine against smallpox. Researchers now use the Vaccinia virus as a model for all poxviruses.
“Smallpox is the most famous and lethal disease caused by a poxvirus, with 1 in 3 infected people dying,” said Professor Wolf, who leads the Molecular Cryo-Electron Microscopy Unit. “But while smallpox has been eradicated in the wild, there are fears that it could be used as a bioweapon. Also, numerous other poxviruses still infect humans and livestock, so further research into how these viruses replicate is essential.”
The scaffold seen in immature poxviruses is of particular interest to scientists, as the structure differs from the protein coats typically seen in viruses.
While most viruses have regular and symmetrical structures, poxvirus scaffolds have roughly spherical honeycomb lattices, which vary in shape between each viral particle.
To determine how these blocks assemble into these spherical honeycomb lattices, the research team used cryo-electron microscopy (cryo-EM) – a technique in which samples are frozen in liquid nitrogen and probed by electrons – to generate 3D images of both single D13 protein trimers and two connected D13 protein trimers, at the highest resolution seen so far.
They found that the two proteins joined together with a slight twist between their trimer axes, creating a curve that is key for forming a spherical shape.
However, when the team used computer modelling to extend the interaction to multiple D13 proteins, they didn’t fit together properly.
“This told us that there must be at least one other way for the two proteins to interact, that we hadn’t yet seen,” said Prof. Hyun.
The researchers also found that when they compared the single D13 protein to the two D13 proteins joined together, a small helix structure at the end of the protein chains had shifted. Previously, the helix structure was buried deep into the pocket where the two proteins interact, suggesting that its repositioning was critical for the two proteins to connect.
To explore the role of the helix structure further, the research team made modified D13 proteins and then used cryo-electron tomography to look at how they self-assembled when placed in solution. When a purification tag was added to the helix, the proteins formed spherical structures similar to the immature poxvirus scaffold. And when the helix was completely removed, the researchers were surprised to see the formation of cylindrical tubes.
Using cryo-EM, the research team were able to capture high-res images of these cylinders and zoom in on the honeycomb structure. They identified a second way for the dimers to interact and found that when they modelled alternating patterns of interaction, the D13 proteins fit together to form the hexagonal honeycomb pattern.
Both modes of how the proteins interacted required the small helix structure to shift, with the proteins then held together by the attraction between positively and negatively charged amino acids.
Overall, the researchers proposed that displacement of the helix was essential for forming poxvirus scaffold, and likely acted as a trigger for the assembly to begin.
“When the poxvirus replicates inside cells, the scaffold forms in association with a lipid membrane,” explained Prof. Wolf. “The helix structure is hydrophobic, which means that it would shift towards to the water-free environment of the lipid membrane, freeing up the pocket where the D13 proteins interact.”
The discovery of the helix’s role in assembly could be a promising new avenue of research for antiviral drug discovery, emphasized Prof. Hyun.
“If we can design a drug that binds really strongly into the pocket where the helix usually sits, it would interfere with formation of the scaffold,” he said. “This is one of my next goals.”
Journal
Nature Communications
DOI
10.1038/s41467-022-29305-5
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Assembly mechanism of the pleomorphic immature poxvirus scaffold
Article Publication Date
31-Mar-2022
COI Statement
The authors declare no competing interests.




