Garden soil houses a variety of bacteria and their natural byproducts — including one that may help halt tumor growth. Lankacidins are molecules that can be isolated from Strepomyces rochei, a common bacterium in soil. In addition to antimicrobial properties, a type of lankacidins, called lankacidin C, can inhibit tumor activity in various cancer cell lines, including leukemia, melanoma, ovarian and breast cancers. Lankacidin C offers a potential foundation on which to design anticancer drugs, but its structure is complicated and difficult to manipulate, according to an international research group. The same group recently identified where antitumor activity is housed on the molecule and has now used that information to simplify lankacidin as a potential starting point to engineer treatments.
Credit: Kenji Arakawa, Hiroshima University
Garden soil houses a variety of bacteria and their natural byproducts — including one that may help halt tumor growth. Lankacidins are molecules that can be isolated from Strepomyces rochei, a common bacterium in soil. In addition to antimicrobial properties, a type of lankacidins, called lankacidin C, can inhibit tumor activity in various cancer cell lines, including leukemia, melanoma, ovarian and breast cancers. Lankacidin C offers a potential foundation on which to design anticancer drugs, but its structure is complicated and difficult to manipulate, according to an international research group. The same group recently identified where antitumor activity is housed on the molecule and has now used that information to simplify lankacidin as a potential starting point to engineer treatments.
They published their results on Jan. 1 in Bioorganic & Medicinal Chemistry.
“Lankacidins have potential antitumor agents, however, their structural modification has somewhat problem due to the presence of complex bicyclic ring in lankacidin antibiotics,” said paper author Kenji Arakawa, associate professor at Hiroshima University’s Graduate School of Integrated Sciences for Life. “Structural modification of lankacidin C, as a mother compound, would be an excellent starting point for enhancing antitumor activity through computational prediction.”
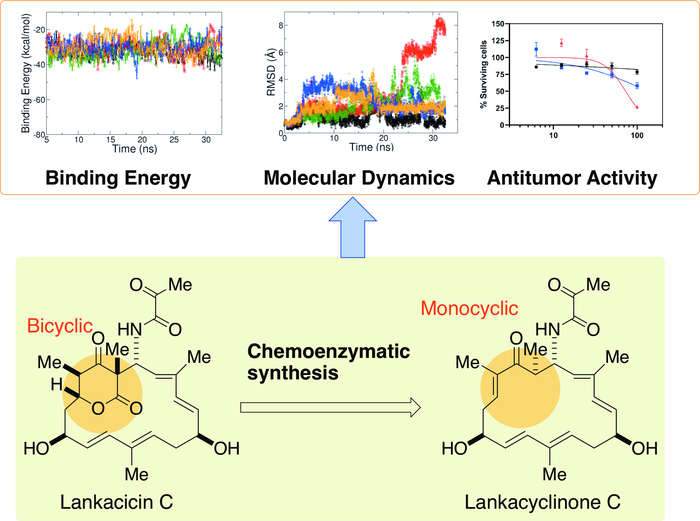
The researchers preliminarily assessed how various components of lankacidin-group antibiotics may contribute to its antitumor activity using a computational model, finding that a structural ring of carbon atoms, called the delta-lactone ring, may not be essential. According to Arakawa, the implication was striking, since the ability to structurally modify lankacidins has been limited by the presence of the delta-lactone ring.
“In this study, we synthesized lankacyclinone C, a novel lankacidin C variant lacking the delta-lactone ring,” Arakawa said. “In doing so, we solved one major issue of structural function in the lankacidin skeleton, the bicyclic structure of the delta-lactone ring, for antitumor activity.”
They used a protein called Orf23 to convert the bicyclic structure with the delta-lactone ring to a monocyclic version. A computational model predicted that the resulting lankacyclinone C, with a simplified ring, would still prove cytotoxic to target cancer cells. Experimental results supported the prediction.
“Rather than bicyclic lankacidins, structurally simple and flexible monocyclic lankacidins may be better substrates for further structural redesigning to improve antitumor activity,” Arakawa said.
According to Arakawa, the researchers plan to further investigate the rational design of molecular compounds with the goal of creating the ultimate antitumor agents.
##
Other contributors include Rukman Muslimin, Natsumi Nishiura and Aiko Teshima, Unit of Biotechnology, Division of Biological and Life Sciences, Graduate School of Integrated Sciences for Life, Hiroshima University, Japan; Kiep Minh Do, Takeshi Kodama and Hiroyuki Morita, Institute of Natural Medicine, University of Toyama, Japan; Cody Wayne Lewis and Gordon Chan, Department of Oncology, Cross Cancer Institute and Cancer Researcher Institute of Northern Alberta, University of Alberta, Canada; and Ahmed Taha Ayoub, Medicinal Chemistry Department, Heliopolis University, Egypt.
Arakawa, Nishiura and Teshima are also affiliated with the Hiroshima Research Center for Healthy Aging, Hiroshima University.
The Japan Society for the Promotion of Science, the Ministry of Scientific Research/Science and Technology Development Fund, the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Izaak Walton Killam Memorial Scholarship, the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health and the Indonesia Endowment Fund for Education supported this work.
About Hiroshima University
Since its foundation in 1949, Hiroshima University has striven to become one of the most prominent and comprehensive universities in Japan for the promotion and development of scholarship and education. Consisting of 12 schools for undergraduate level and 4 graduate schools, ranging from natural sciences to humanities and social sciences, the university has grown into one of the most distinguished comprehensive research universities in Japan.
English website: https://www.hiroshima-u.ac.jp/en
Journal
Bioorganic & Medicinal Chemistry
DOI
10.1016/j.bmc.2021.116551
Article Title
Chemoenzymatic synthesis, computational investigation, and antitumor activity of monocyclic lankacidin derivatives
Article Publication Date
1-Jan-2022



