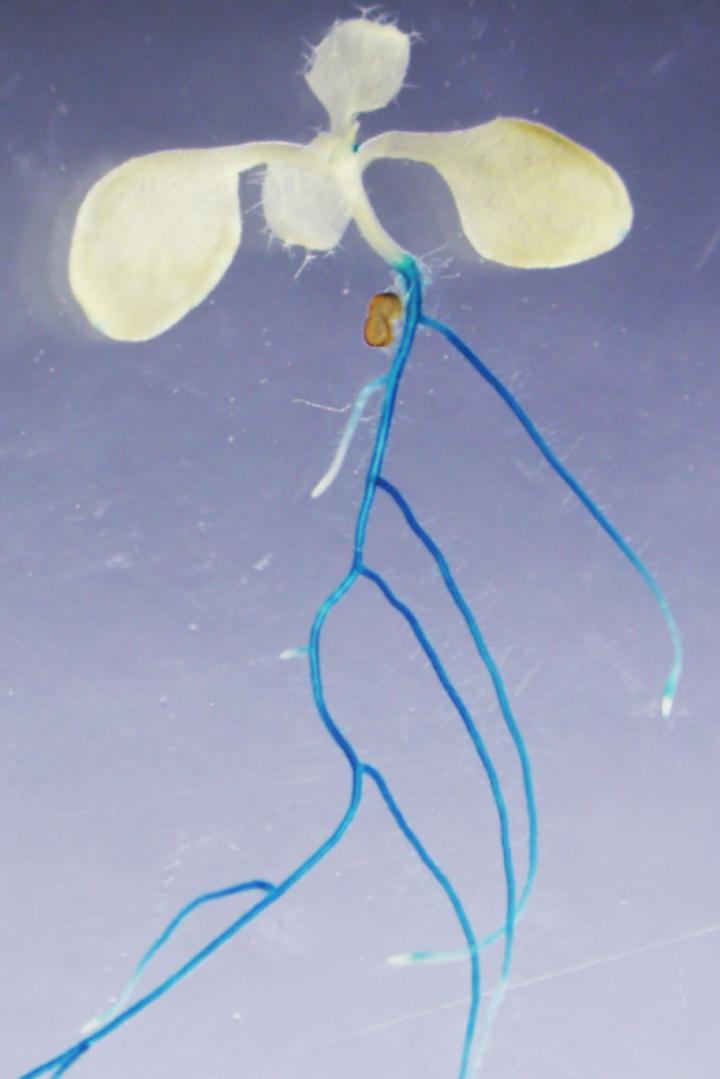
Nitrates are critical for the growth of plants, so plants have evolved sophisticated mechanisms to ensure sufficient nitrate uptake from their environments. In a new study published in Nature Plants, researchers at Nagoya University, Japan, have identified a plant enzyme that is key to activating a nitrate uptake mechanism in response to nitrogen starvation. This finding explains how plants meet their needs in challenging environments, opening doors to improving agriculture in such environments.
When nitrate levels are plentiful in a plant’s environment, a plant can achieve adequate nitrate uptake levels by relying on what plant biologists call a “low-affinity transport system.” But when nitrates become scarce in a plant’s local environment, it may need to switch to a more powerful nitrate uptake mechanism known as a “high-affinity transport system.” In Arabidopsis plants, which frequently serve as model organisms for plant biology research, the NRT2.1 protein plays an important role in the high-affinity transport system. Interestingly, when Arabidopsis plants synthesize the NRT2.1 protein, they initially produce an inactive protein that can later be activated when the high-affinity transport system is needed.
This synthesis of a nonfunctioning protein that can later be activated intrigued Dr. Yoshikatsu Matsubayashi of Nagoya University, but he sees a certain logic in this preparatory protein synthesis; he notes, “Proteins cannot be synthesized when the nitrogen deficiency occurs.” In other words, plants need to synthesize the proteins in a high-affinity transport system before a nitrogen deficiency necessitates the use of those proteins, because nitrogen deficiency itself makes it difficult to synthesize new proteins. In order to better understand this remarkable system, Dr. Matsubayashi and his colleagues set out to identify the protein that activates NRT2.1 in response to nitrogen starvation.
Previous studies had shown that a peptide called CEP found in plant roots plays an important role in activating biochemical pathways that respond to nitrogen starvation, so the researchers focused their investigation on the CEP and its downstream CEPD pathway. Their experiments soon drew their attention to a protein called At4g32950. The researchers found that this protein responds to nitrogen starvation by activating the NRT2.1 protein. It achieves this activation by removing a phosphate group from a specific location on the NRT2.1 protein, so the investigators decided to give the At4g32950 protein a new name: CEPD-induced phosphatase, or “CEPH” for short.
CEPH is found mainly in the cells close to the surface of the Arabidopsis plant’s roots, which is an optimal location for activating a system that evolved for rapid nitrate uptake from the environment. As expected, using laboratory methods to inactivate the gene that encodes CEPH impaired the ability of Arabidopsis plants to use the high-affinity transport system for rapid nitrate uptake, and this meant that the modified plants had lower internal nitrate levels and grew to smaller sizes.
Collectively, these results indicate that CEPH plays a critical role in responding to nitrogen starvation through its activation of the NRT2.1 protein. Dr. Matsubayashi sees considerable potential utility in CEPH as a genetic engineering tool, as he notes, “Artificially enhancing CEPH activity could enable scientists to create plants that grow even in soils with low nutrient levels.” Such findings could change the way agriculture and food security are handled.
###
The paper, “A type 2C protein phosphatase activates high-affinity nitrate uptake by dephosphorylating NRT2.1,” was published in the journal Nature Plants on March 8, 2021 at DOI: 10.1038/s41477-021-00870-9.
About Nagoya University, Japan
Nagoya University has a history of about 150 years, with its roots in a temporary medical school and hospital established in 1871, and was formally instituted as the last Imperial University of Japan in 1939. Although modest in size compared to the largest universities in Japan, Nagoya University has been pursuing excellence since its founding. Six of the 18 Japanese Nobel Prize-winners since 2000 did all or part of their Nobel Prize-winning work at Nagoya University: four in Physics – Toshihide Maskawa and Makoto Kobayashi in 2008, and Isamu Akasaki and Hiroshi Amano in 2014; and two in Chemistry – Ryoji Noyori in 2001 and Osamu Shimomura in 2008. In mathematics, Shigefumi Mori did his Fields Medal-winning work at the University. A number of other important discoveries have also been made at the University, including the Okazaki DNA Fragments by Reiji and Tsuneko Okazaki in the 1960s; and depletion forces by Sho Asakura and Fumio Oosawa in 1954.
Website: http://en.
Media Contact
Yoshikatsu Matsubayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.