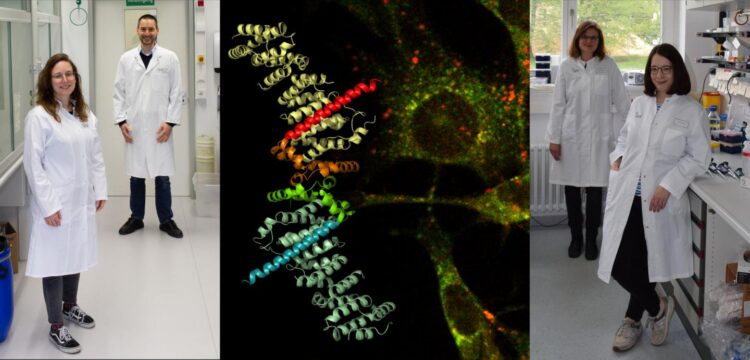
Mutations can disrupt protein binding through a “burr effect” thus interfering with the regulation of cell growth
Credit: Kümmel team/Oeckinghaus team
Tuberous Sclerosis Complex (TSC) affects between one and two of every 10,000 new-born babies. This genetic disease leads to the formation of benign tumours which can massively impair the proper functioning of vital organs such as the kidneys, the liver and the brain. The disease affects different patients to varying degrees and is triggered by mutations in one of two genes, the TSC1 or TSC2 gene. An interdisciplinary team of researchers led by biochemists Prof. Daniel Kümmel and Dr. Andrea Oeckinghaus from the University of Münster (Germany) examined the “tumour suppressor protein TSC1” and, for the first time, gained insights into its hitherto unclear functions. The team identified a new mechanism, in a central cellular process, which regulates cell growth. The results can also help in understanding how Tuberous Sclerosis Complex arises. The results of the study have now been published in the journal Molecular Cell (advance publication online).
Mutations concern a “burr effect”
The TSC1 protein and the TSC2 protein together form the TSC protein complex. This has the task of controlling cell growth and, as a result, of suppressing the emergence of tumours – hence the term “tumour suppressor”. Previously, it had largely been unclear what the functions of TSC1 were. Similarly, little was known about the mechanism which is affected by certain mutations in the TSC1 gene in the occurrence of the disease. The researchers have now found out that one part of the TSC1 protein, a so-called domain, can bind to the surfaces of lysosomal membranes. Lysosomes are small compartments in the interior of the cell, surrounded by a membrane, which contain digestive enzymes. On their surface there are certain control centres which are important for the regulation of cell growth. The TSC1 protein ensures that the entire TSC complex arrives at these control centres and prevents any uncontrolled cell growth by inhibiting the activity of an important signal protein called “mTOR” – “Mechanistic Target of Rapamycin”.
“In this process,” Daniel Kümmel explains, “TSC1 uses a strategy reminiscent of the principle of the burr. The burr’s individual hooks stick to material only very weakly, but lots of hooks together provide a firm attachment.” The study shows that TSC1’s individual membrane binding domain only forms a weak attachment. However, a strong attachment becomes possible with a controlled aggregation of a large number of TSC1 molecules. “Mutations occur particularly frequently in the membrane binding domain,” says Andrea Oeckinghaus. “We assume that some of the pathogenic effects can now be explained by a loss of the correct localization of the TSC complex.”
Membrane component regulates cell growth
Another discovery the team made is that the properties of the membrane surface can influence the way the TSC complex functions and, as a result, the growth processes in the cell. More precisely, the team identified a special component in the membrane: a lipid called phosphatidylinositol 3,5-bisphosphate (PI3,5P2), which is necessary for the activity of the TSC complex. Depending on how often it occurs on the membrane’s surface, it has different effects on the activity. “As the production and breakdown of this lipid are regulated,” says Daniel Kümmel, “this opens up entirely new perspectives on how cell growth can be controlled. This means that our results are an exciting starting point for further studies.”
The team of researchers used a broad range of methods in its investigations, starting from approaches using structural biology and biochemistry, and also involving cell-biological experiments. “The resulting insights into mechanistic and physiological aspects were only possible through our interfaculty collaboration,” says Kümmel.
The work was carried out jointly by the team headed by Dr. Andrea Oeckinghaus (Münster University Faculty of Medicine and Münster University Hospital, Institute of Molecular Tumour Biology) and the team led by Prof. Daniel Kümmel (Münster University Faculty of Chemistry and Pharmacy, Institute of Biochemistry). Important contributions were made by partners at the Max Planck Institute for the Biology of Ageing, Cologne (team headed by Dr. Constantinos Demetriades) and at the Max Planck Institute for Molecular Physiology, Dortmund (team led by Prof. Stephan Raunser).
###
Media Contact
Dr. Andrea Oeckinghaus
[email protected]
Original Source
https:/
Related Journal Article
http://dx.