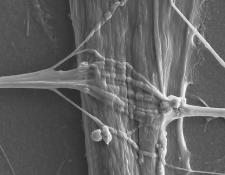
Credit: Dr. Pieter Baatsen, LiMoNe, Research Group Molecular Neurobiology and Katarina Stoklund Dittlau, Laboratory of Neurobiology at VIB-KU Leuven Center for Brain and Disease Research
Skeletal muscles enable voluntary movements and are controlled by a special type of neurons called motor neurons, which make direct contact with skeletal muscles through so-called neuromuscular junctions (NMJs). It is through NMJs that skeletal muscles receive signals making them contract or relax. In certain neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), NMJs are destroyed, leading to progressive muscle weakness and ultimately death. Treatments for ALS mainly focus on alleviating symptoms but cannot stop or reverse its disease progression. To find more effective treatments, researchers require accurate and easily accessible lab-based models for ALS to understand its causes and to develop and test new therapies. One step in this direction was made by Ludo Van Den Bosch ([email protected]) and colleagues in Belgium, who generated NMJs outside the human body in a so-called microfluidic device. In this sophisticated model, human motor neurons, which were derived from ALS patients, via induced pluripotent stem cells engineered from their own skin cells, and human skeletal muscle cells from healthy donors grew in separate mini-chambers on opposite sides of the device, whereby tiny channels connected the two chambers. Excitingly, with time, the neurons started to send connections, called axons, through the channels to form NMJs, which were able to transmit signals from the neurons to the muscle cells, similar to NMJs in the human body. However, when comparing motor neurons from ALS patients with healthy motor neurons in this setup, it was clear that ALS motor neurons sent less axons across the channels and formed less NMJs with the muscle cells. In addition, ALS motor neurons regenerated damaged axons less efficiently. Encouragingly, ALS motor neurons could be pushed to grow more axons, reaching levels similar to healthy motor neurons, by adding the chemical Tubastatin A to the cultures. Further studies will show how Tubastatin A, which inhibits a certain class of proteins in the cell, promotes axon growth in ALS motor neurons, and if similar effects can be achieved in animal models and ultimately in ALS patients. This new miniaturized model of NMJ formation, recently published in Stem Cell Reports, will find broad application in studying motor neuron pathology and the discovery of potential therapeutics in ALS.
###
Media Contact
Kym Kilbourne (ISSCR)
[email protected]
Related Journal Article
http://dx.




