Credit: Image created by Patricia Pereira, PhD, Research Associate at Memorial Sloan Kettering Cancer Center
Reston, VA–Immuno-positron emission tomography (PET) imaging can provide early insight into a tumor’s response to targeted therapy, allowing physicians to select the most effective treatment for patients who have cancer. The new research was published in the March issue of The Journal of Nuclear Medicine.
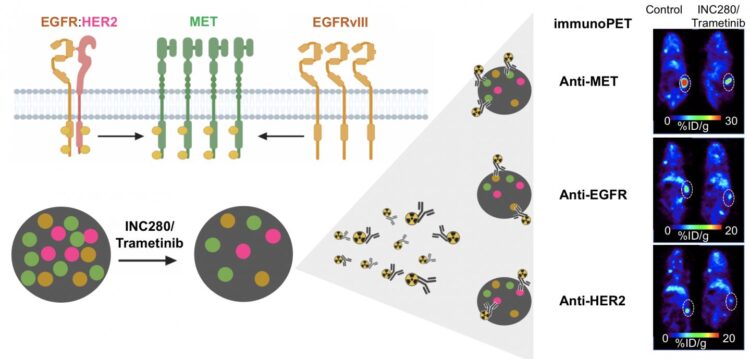
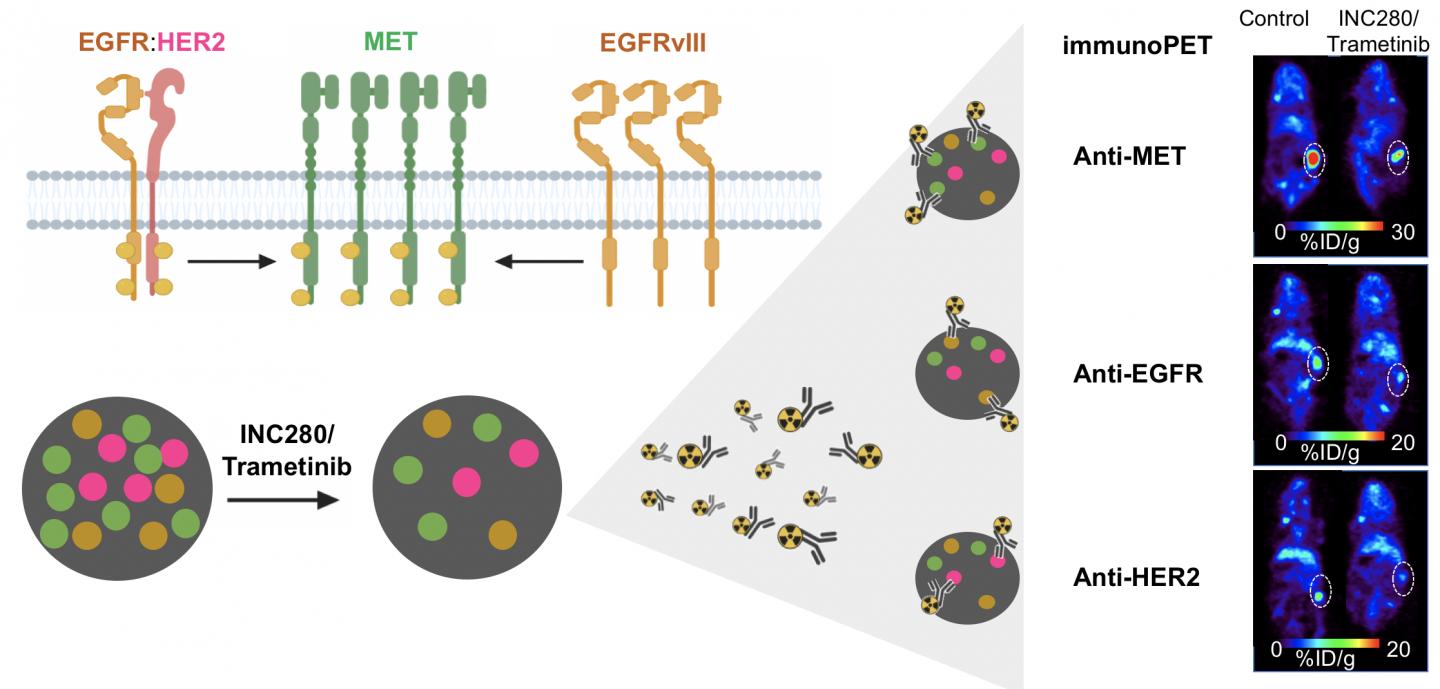
The research showed that immuno-PET successfully visualizes changes in different cancer receptors (receptor tyrosine kinases, or RTKs) within tumors during targeted therapies. This gives physicians a tool that can be used to evaluate the effectiveness of a treatment soon after its administration.
“When healthy cells turn into cancer cells, there is a disruption in the RTK signaling. This makes RTKs a valuable therapeutic and imaging target,” said Patricia Pereira, Ph.D., a research associate at Memorial Sloan Kettering Cancer Center in New York, New York. “Techniques that allow for real-time monitoring of RTK dynamics, such as immuno-PET, could be very beneficial in informing treatment choice and predicting response.”
Immuno-PET uses a “tracer” to follow an antibody directed to a specific tumor. This allows physicians to obtain images of events happening at the tumor site and provides information into whether the tumor responds to the treatment. The physician can then visualize how the tumor is responding.
In this study, researchers used immuno-PET and three different antibodies to visualize three RTKs (MET, EGFR, and HER2) in a kidney tumor. Their results confirmed that immuno-PET visualizes RTKs in ways that determine the level of protein within a tumor. After administering a treatment, immuno-PET can detect changes in RTK levels that indicate whether a tumor is responsive to that treatment.
“Precision medicine involves the identification of certain gene mutations and expressions, as well as other features, that contribute individual tumor signatures,” noted Pereira. “Our study shows that immuno-PET is a powerful technique to document RTK changes and predict tumors’ response to targeted therapies.”
###
This study was made available online in July 2020 ahead of final publication in print in March 2021.
The authors of “Immuno-PET Detects Changes in Multi-RTK Tumor Cell Expression Levels in Response to Targeted Kinase Inhibition,” include Patricia M.R. Pereira and Jalen Norfleet, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, New York; Jason S. Lewis, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, New York, and Molecular Pharmacology Program and Radiochemistry and Molecular Imaging Probes Core, Memorial Sloan Kettering Cancer Center, and Departments of Pharmacology and Radiology, Weill Cornell Medical College, New York, New York; and Freddy E. Escorcia, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Visit JNM’s new website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed.
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected]
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed more than 11 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging–precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit http://www.
Funders: Onartuzumab was provided by Genentech. The Radiochemistry and Molecular Imaging Probe Core and the Antitumor Assessment Core were supported by NIH grant P30 CA08748. This study was supported in part by the Geoffrey Beene Cancer Research Center of MSKCC (Jason Lewis), NIH NCI grant R35 CA232130 (Jason Lewis), NIH NCI grant ZIA BC 011800 (Freddy Escorcia), Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Center for Experimental Therapeutics of Memorial Sloan Kettering Cancer Center. Freddy Escorcia is supported by the American Board of Radiology Leonard B. Holman Research Pathway and the Clinical Investigator Development Program of NCI and NIH. Patricia Pereira is supported by the Tow Foundation Postdoctoral Fellowship from the MSKCC Center for Molecular Imaging and Nanotechnology and the Alan and Sandra Gerry Metastasis and Tumor Ecosystems Center of MSKCC. No other potential conflict of interest relevant to this article was reported.
Media Contact
Rebecca Maxey
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.