Credit: ZHANG Xiaolong
In 1952, Alan Turing, the father of computer science and artificial intelligence, proposed that certain repetitive natural patterns may be produced by the interaction of two specific substances through the “reaction-diffusion” process. In this system, activator promotes the reaction and inhibitor inhibits the reaction. When the two meet, the reaction diffuses. When the difference in diffusion coefficient between the two reaches a certain level, the high diffusion ratio between them will cause the system imbalance and induce the formation of periodic complex patterns.
“Turing structure” exists widely in nature, such as the body patterns of zebras, the phyllotaxis of sunflowers, the follicle spacing of mouse hairs and others. However, it is difficult to construct a Turing structure in a manmade chemical system since the difference in diffusion coefficients of substances is small.
Recently, the research group of Prof. GAO Minrui from the University of Science and Technology of China created the Turing structure on inorganic transition metal chalcogenides with the “reaction-diffusion” process for the first time. Results were published in German Applied Chemistry and was selected as Hot Paper and Back Cover.
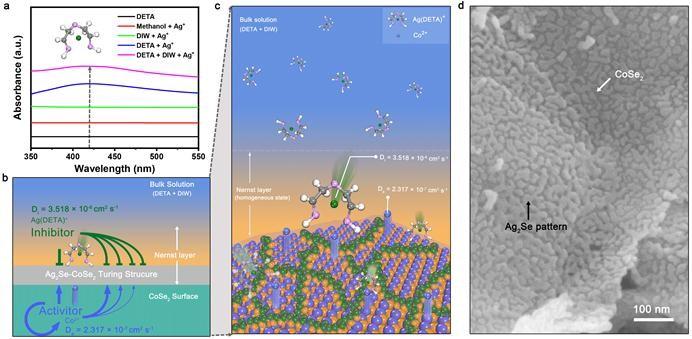
In the binary solution of diethylenetriamine (DETA) and water, Ag+ will react with DETA to form Ag(DETA)+. At the same time, Co2+ overflows from the surface of the cobalt diselenide (CoSe2) nanobelt. Ag(DETA)+ is the inhibitor and Co2+ is the activator in this system. When the rapidly diffused Ag (DETA)+ reaches the Nernst layer on the CoSe2 surface, it interacts with the activator Co2+ diffused on the CoSe2 surface, and finally forms a complex and beautiful Ag2Se Turing pattern on the CoSe2 surface.
The study found that this multi-interface Turing structure material, Ag2Se-CoSe2, was an efficient oxygen evolution (OER) electrocatalyst. The OER activity of Ag2Se-CoSe2 was linearly related to the interface length of the Turing structure. The rich interface structure and the optimized OER intermediate adsorption energy at the interface structure conspired to bring about its high activity.
This study uses the “reaction-diffusion” theory to construct complex Turing structures on inorganic nanostructured materials for the first time, and provides new ideas for the design of cheap catalysts with higher performance.
This research employed the “reaction-diffusion” theory to build a complex Turing structure on inorganic nanostructured materials for the first time, and provided a new path for designing cheaper catalysts with higher performance.
###
Media Contact
Jane FAN Qiong
[email protected]
Related Journal Article
http://dx.




