Scientists engineer a light-activated protein that can be used to study single synapses of neurons
Credit: Hideji Murakoshi
Neurons are the primary cells of the nervous system, and the signals that are transmitted between them are responsible for all our actions and our cognitive ability. In particular, learning and memory are believed to be associated with a process called “long-term potentiation,” which is the strengthening of connections between specific neurons via continued signal transmission through “synapses” (small gaps between neurons). Long-term potentiation can change the connection between neurons via synapses–by changing their size and composition. Understanding how long-term potentiation occurs can be valuable to clarifying how our brain learns and retains new knowledge. A team of scientists from Japan has now made significant strides in the understanding of long-term potentiation. Read on to know how!
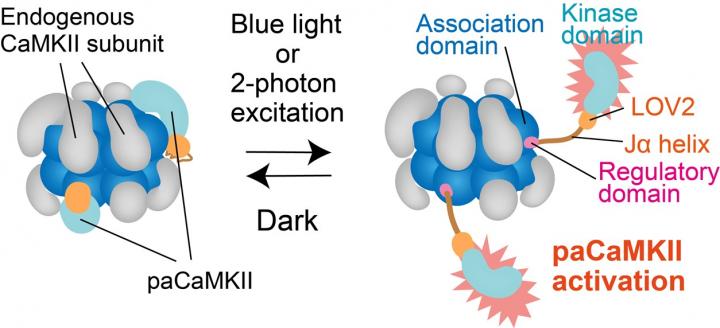
One way to study long-term potentiation is by using “optogenetics,” i.e., activating neurons and monitoring their responses upon light stimulation. Optogenetics allows scientists to activate single neurons and dissect how neurons work in neuronal networks. As such, optogenetics is a revolutionary advancement in neuroscience research, but optogenetic tools to modify single synapses (spines) have not been developed until now. This is an issue because neuronal signaling pathways may have spine-specific effects. In particular, the protein “CaMKII,” critical for long-term potentiation, is activated by the molecule “glutamate” in a spine-specific way, but exactly what happens at the synapses during activation remains a mystery.
Fortunately, a research team at National Institute of Physiological Sciences, Japan, led by Dr. Hideji Murakoshi, resolved this issue. The team fused CaMKII with a specific domain of a plant photoreceptor (a type of cell that responds to light). This domain called “LOV2-Jα” caused CaMKII to become sensitive to light, after which they expressed this new photoactivatable CaMKII in different types of isolated neurons and in living mice. Their findings are recently published in the journal Nature Communications.
“We were very excited to find that activating CaMKII triggers some important effects, specifically recruiting receptors that cause a chain reaction that then leads to long-term potentiation,” Dr. Murakoshi explains. The process physically changes the dendritic spines, expanding them, an outcome that the scientists also observed in their experiments. Importantly, all that was needed for this process to happen was CaMKII activation–to put it in scientific terms, CaMKII activation was sufficient for long-term potentiation of individual dendritic spines, which had not been demonstrated before. The team also used light-based imaging technology and the light-activated CaMKII to determine what signaling molecules are activated during long-term potentiation. All of these findings combine to provide a better picture of how long-term potentiation occurs at the synapse level.
“In addition to the valuable information we uncovered about an important neurological process, our light-activated CaMKII is a major addition to existing optogenetic tools,” Dr. Murakoshi comments when asked about the significance of their work. “We’ve created something that can be used to manipulate neuronal signaling and investigate synaptic plasticity–or the physiological changes that happen at individual synapses during events like memory formation.”
The scientists are optimistic that with further development, the ability to manipulate synapses also has important implications for the treatment of brain diseases (such as autism)–a remarkable feat for neuroscience!
###
Media Contact
Hideji Murakoshi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




