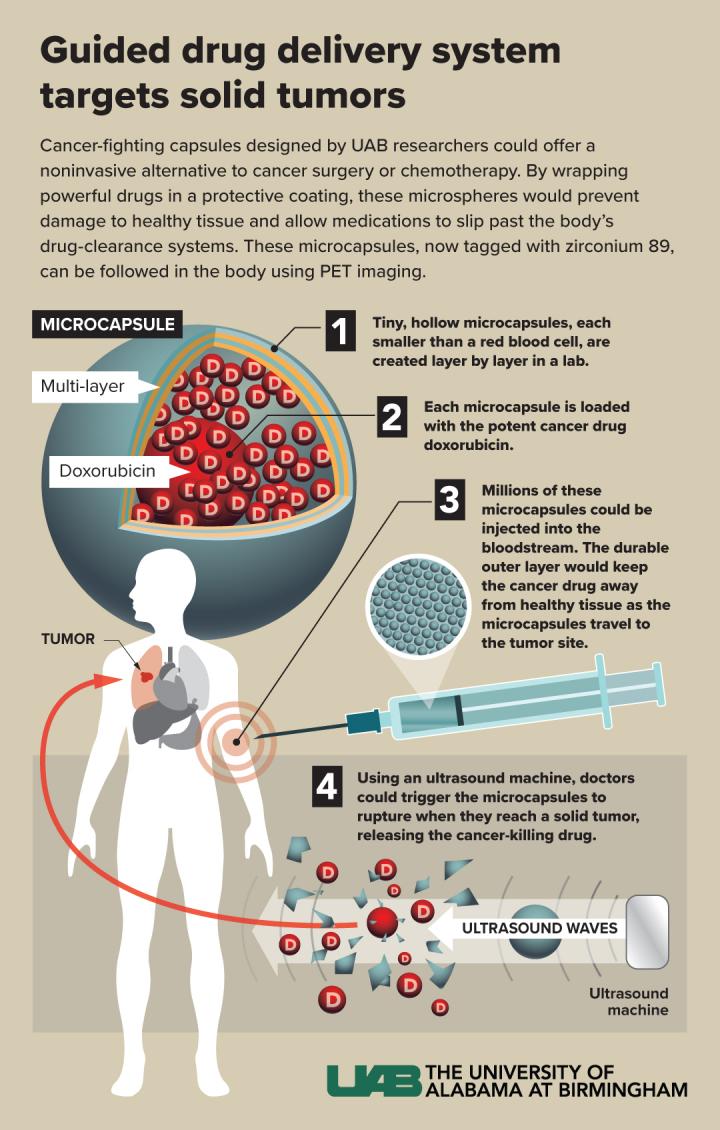
If these hollow capsules are modified to target a solid tumor, PET imaging and therapeutic ultrasound can be used to rupture them and release an anticancer drug at ground zero.
Credit: UAB
BIRMINGHAM, Ala. – University of Alabama at Birmingham polymer and radionuclide chemists report what they say “may represent a major step forward in microcapsule drug delivery systems.”
The UAB microcapsules — labeled with radioactive zirconium-89 — are the first example of hollow polymer capsules capable of long-term, multiday positron emission tomography, or PET, imaging in vivo. In previous work, UAB researchers showed that the hollow capsules could be filled with a potent dose of the cancer drug doxorubicin, which could then be released by therapeutic ultrasound that ruptures the microcapsules.
PET imaging with zirconium-89 — which has a half-life of 3.3 days — allowed the capsules to be traced in test mice up to seven days. The major step forward in the current research was covalent incorporation of a zirconium chelator, deferoxamine or DFO, into the layers of the microcapsules. This increased the tight binding of the positron emitter zirconium-89 onto the microcapsule wall.
“The produced systems represent an example of an advanced theranostic agent with the possibility to combine controlled drug delivery with stable PET imaging capabilities,” said Eugenia Kharlampieva, Ph.D., and Suzanne Lapi, Ph.D., co-senior authors. “We believe this system also provides the groundwork for a universal drug delivery carrier that can be coupled with advanced targeted treatments, such as patient-tailored gene therapeutics, and can lead to the advancement of human health through molecularly targeted, image-guided precision drug delivery.”
This drug delivery system — after surface modifications to boost targeting capabilities — could offer a non-invasive alternative to cancer surgery or systemic chemotherapy for solid tumors. The term theranostic, a portmanteau of the words therapy and diagnostic, refers to nanoparticles or microcapsules that can double as diagnostic imaging agents and as therapeutic drug-delivery carriers.
The relative paucity of zirconium-89 functionalized particles, Kharlampieva and Lapi suggest, may be due to both the scarcity of laboratories able to work with this isotope and the challenges associated with its stable incorporation into a polymeric drug carrier. At UAB, Kharlampieva is a professor in the UAB College of Arts and Sciences Department of Chemistry and a co-director of the UAB Center for Nanomaterials and Biointegration. Her polymer chemistry lab has pioneered research into hollow microcapsules that are built with alternating layers of biocompatible tannic acid and poly(N-vinylpyrrolidone), or TA/PVPON. The layers are formed around a sacrificial core that is dissolved after the layers are complete. In the current research, the chelator DFO was covalently linked to the PVPON, and the capsules were composed of alternating layers of TA and PVPON-DFO, for a total of six or 12 bilayers.
Lapi is professor of radiology and vice chair of Translational Research, and she is the director of the UAB Cyclotron Facility, which produces both clinical and investigational radionuclides. Lapi also directs the Radiochemistry Laboratory and the Division of Advanced Medical Imaging Research.
The UAB researchers found that (TA/PVPON-DFO) six-bilayer capsules retained, on average, 17 percent more zirconium-89 than their (TA/PVPON) counterparts, evidence that linking DFO to PVPON provides stable chelation. In vivo PET imaging of mice showed excellent stability and imaging contrast that was still present seven days post-injection. The capsules accumulated primarily in the spleen, liver and lungs, and there was negligible accumulation in the femur. Since the femur is the site where free zirconium-89 accumulates, this lack of accumulation in the femur confirmed stable binding of the radiotracer to the capsule. Finally, applying therapeutic levels of ultrasound to the zirconium-functionalized capsules released the anticancer drug doxorubicin — loaded inside the microcapsules — in therapeutic amounts.
Thus, the microcapsules overcome three limitations seen in the majority of current PET-guided theranostic agents: 1) poor retention of radiometal over time, 2) low drug-loading capacities, and 3) time-limited PET imaging capability.
###
Co-first authors of the study, “Multilayer microcapsules with shell-chelated 89Zr for PET imaging and controlled delivery,” published in the journal ACS Applied Materials & Interfaces, were Veronika Kozlovskaya, Ph.D., and Aaron Alford, Ph.D., UAB Department of Chemistry.
Other co-authors with Kharlampieva, Lapi, Kozlovskaya and Alford are Maksim Dolmat and Racquel Caviedes, UAB Department of Chemistry; and Maxwell Ducharme and Lauren Radford, UAB Department of Radiology.
Support came from National Science Foundation Division of Materials Research award 1608728 and National Science Foundation Major Research Instrumentation Program award 1828232. Imaging studies were also supported through the O’Neal Comprehensive Cancer Center at UAB grant P30CA013148.
Media Contact
Jeff Hansen
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




