Credit: ©Science China Press
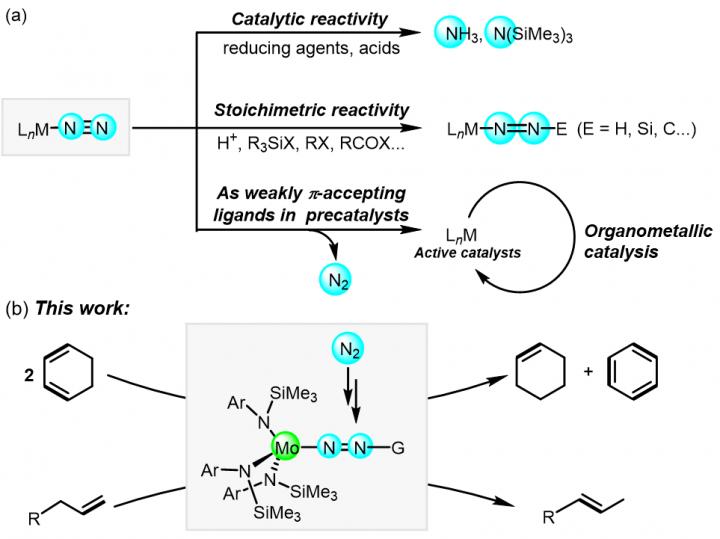
Dinitrogen (N2) fixation is considered as one of the most essential tasks in basic science, providing straightforward methods to produce ammonia and nitrogen-containing molecules. Exploring the reactivity of N2 units of transition metal-nitrogen complexes is of great significance and challenging in the chemistry. Since the first Ru-N2 complex was prepared in 1965, important progress has been made in the synthesis and reactivity of transition metal nitrogen complexes. In many cases, terminal end-on M-N2 complexes as the most prevalent bonding mode were proved to be effective to catalyze reductive reactions of N2 to afford ammonia or silylamines; moreover, due to the nucleophilicity of dinitrogen moiety, the activated dinitrogen ligands in M-N2 (M = Mo, W, Fe, Co) complexes were transformed into N-containing organic compounds with carbon-based electrophiles. On the other hand, late-transition metal-N2 complexes (M = Co, Ru, Ir, Fe) have also been reported as precatalysts for organometallic transformations including cycloaddition and hydrofunctionalization of olefins, semihydrogenation of alkynes, transfer hydrogenation of ketones and acceptorless dehydrogenation of alcohols. In these systems, dinitrogen (N2) as weakly π-accepting ligand to stabilize highly reactive and low valence-electron species, was proved not to be involved in the catalytic processes. The status quo urges exploration on the potential reactivity of coordinated N2 units in organometallic catalysis, which will inspire new strategies for catalytic conversions of N2.
More recently, the research group of Professor Zhang-Jie Shi from Fudan University reported the synthesis, structural characterization and catalytic reactivity exploration of molybdenum-nitrogen complexes supported by monodentate arylsilylamino ligand in the National Science Review (NSR). The results showed that complexes [Ar(TMS)N]3MoN2Mg(THF)2[N(TMS)Ar] (A) and [Ar(TMS)N]3MoN2TMS (B) could be used as effective catalysts for the disproportionation of cyclohexadienes to generate cyclohexene and benzene. It was noteworthy that those catalysts remained intact after complete conversion of the substrate, suggesting that the activated -N2 units were retained during catalysis. Other complexes such as Mo[N(TMS)Ar]3, ClMo[N(TMS)Ar]3, Mg[N(TMS)Ar]2 or Li[N(TMS)Ar] failed for promoting such a disproportionation efficiently. Kinetic studies showed that the initial rate of disproportionation depend on the concentration of catalyst A was the first order, providing the evidence of A not as a precatalyst in the transformation; the authors proposed that the Mo-N=N unit in the molybdenum-nitrogen complex played a key role in the reaction through the LLHT process. In addition, applying the LLHT-reverse LLHT reaction process to terminal olefins such as allylbenzene or 1-hexene the isomerization indeed took place in high efficiency. Kinetic studies indicated the C-H cleavage was not involved in the rate-determining step.
The research results showed that molybdenum-nitrogen complexes possessed high catalytic activity in organometallic catalytic conversion, which may suggest new approaches to participate in catalytic systems for nitrogen ligands, and afforded new perspectives to examine coordination modes between metal centers and nitrogen ligands.
###
See the article:
Silylamido supported dinitrogen heterobimetallic complexes: Syntheses and their catalytic ability
https:/
Media Contact
Zhang-jie Shi
[email protected]
Related Journal Article
http://dx.




