Researchers from the University of Tsukuba identify the active nitrogen atoms in the carbon catalyst of a technology that will help optimize a proposed renewable energy storage technology
Credit: University of Tsukuba
Tsukuba, Japan – Proton-exchange membrane (PEM) fuel cells are an energy storage technology that will help lower the environmental footprint of transportation. These fuel cells make use of a chemical reaction known as oxygen reduction. This reaction needs a low-cost catalyst for widespread commercial applications. Nitrogen-doped carbon is one such catalyst, but the chemical details of how nitrogen doping works are rather controversial. Such knowledge is important to improving the function of PEM fuel cells in future technologies.
In a study recently published in Angewandte Chemie International Edition, researchers from the University of Tsukuba reported chemical details for optimizing the oxygen reduction reaction in PEM fuel cells in acidic conditions. This configuration helps the carbon catalyst adsorb oxygen in a way that enables the fuel cell to function.
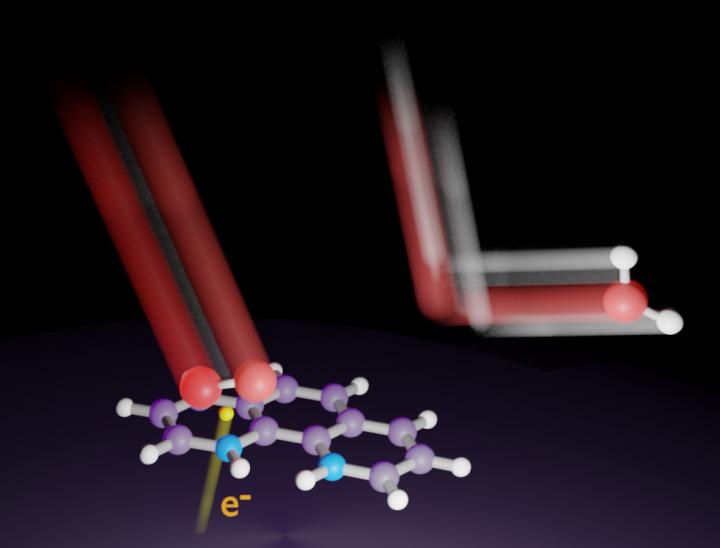
Nitrogen can adopt various bonding configurations, such as pyridinic, in nitrogen-doped carbon catalysts. For years, researchers have tried to determine which bonding configurations are the source of electrolytic activity in PEM fuel cells. The results of such studies may be unclear unless the reaction mechanisms are clarified with controlled bonding and crystallographic orientation of the nitrogen atom on the catalysts.
“We deposited seven nitrogenous molecules onto a paracrystalline carbon black catalyst to make model catalysts with homogeneous structures,” says lead author Professor Kotaro Takeyasu. “We found that 1,10-phenanthroline, with two pyridinic nitrogen atoms at the armchair edges of the catalyst, had the highest activity with reference to current density.”
Sulfuric acid fully acidifed the nitrogen atoms in the catalyst. Upon applying an appropriate voltage under oxygen-saturated conditions, the protonated nitrogen atoms in the catalyst were reduced. This was attributable to the simultaneous oxygen adsorption, because there was no reduction in nitrogen-saturated conditions.
“Density functional theory calculations also indicate that oxygen adsorption promotes the reduction of fully protonated nitrogen atoms,” explains senior author, Professor Junji Nakamura. “Thus, oxygen absorbs onto the catalyst and at the same time, the nitrogen atoms are reduced for additional catalytic cycles.”
Current PEM fuel cells use platinum catalysts. Because platinum is a rare metal it is not a realistic option for commercial applications in the long term. Thus, platinum catalysts will not enable PEM fuel cells to contribute to a low-carbon economy. The findings described here will help researchers improve the performance of carbon-based catalysts for PEM fuel cells and improve the sustainability of transportation.
###
The article, “Role of pyridinic nitrogen in the mechanism of the oxygen reduction reaction on carbon electrocatalysts,” was published in Angewandte Chemie International Edition at DOI: 10.1002/anie.202014323
Media Contact
Naoko Yamashina
[email protected]
Related Journal Article
http://dx.




