Rice engineers’ reactor converts gas directly into acetic acid
Credit: Wang Group/Senftle Group/Rice University
HOUSTON – (Jan. 11, 2021) – A sweet new process is making sour more practical.
Rice University engineers are turning carbon monoxide directly into acetic acid — the widely used chemical agent that gives vinegar its tang — with a continuous catalytic reactor that can use renewable electricity efficiently to turn out a highly purified product.
The electrochemical process by the labs of chemical and biomolecular engineers Haotian Wang and Thomas Senftle of Rice’s Brown School of Engineering resolves issues with previous attempts to reduce carbon monoxide (CO) into acetic acid. Those processes required additional steps to purify the product.
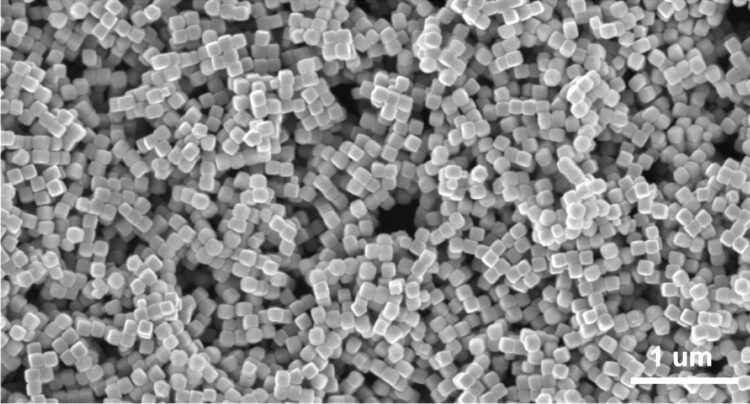
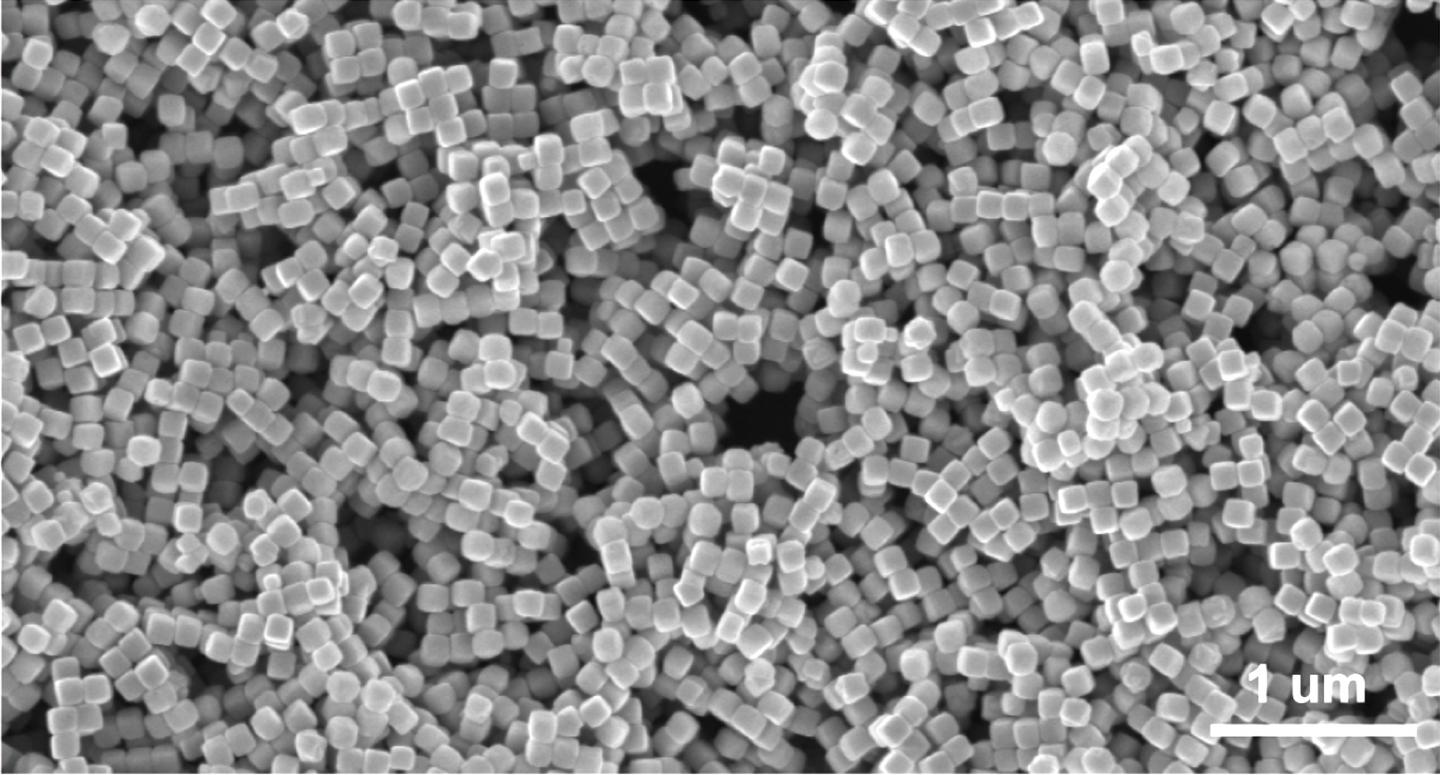
The environmentally friendly reactor uses nanoscale cubes of copper as the primary catalyst along with a unique solid-state electrolyte.
In 150 hours of continuous lab operation, the device produced a solution that was up to 2% acetic acid in water. The acid component was up to 98% pure, far better than that produced through earlier attempts to catalyze CO into liquid fuel.
Details appear in the Proceedings of the National Academy of Sciences.
Along with vinegar and other foods, acetic acid is used as an antiseptic in medical applications; as a solvent for ink, paint and coatings; and in the production of vinyl acetate, a precursor to common white glue.
The Rice process builds upon the Wang lab’s reactor to produce formic acid from carbon dioxide (CO2). That research established an important foundation for Wang, recently named a Packard Fellow, to win a $2 million National Science Foundation (NSF) grant to continue exploring the conversion of greenhouse gases into liquid fuels.
“We’re upgrading the product from a one-carbon chemical, the formic acid, to two-carbon, which is more challenging,” Wang said. “People traditionally produce acetic acid in liquid electrolytes, but they still have the issue of low performance as well as separating the product from the electrolyte.”
“Acetic acid is typically not synthesized, of course, from CO or CO2,” Senftle added. “That’s the key here: We’re taking waste gases we want to mitigate and turning them into a useful product.”
It took a careful coupling between the copper catalyst and solid electrolyte, the latter carried over from the formic acid reactor. “Sometimes copper will produce chemicals along two different pathways,” Wang said. “It can reduce CO into acetic acid and alcohols. We engineered copper cubes dominated by one facet that can help this carbon-carbon coupling, with edges that direct the carbon-carbon coupling towards acetic acid instead of other products.”
Computational models by Senftle and his team helped refine the cubes’ form factor. “We were able to show there are types of edge on the cube, basically more corrugated surfaces, that facilitate breaking certain C-O bonds that steer the products one way or the other,” he said. “Having more edge sites favors breaking the right bonds at the right time.”
Senftle said the project was a great demonstration of how theory and experiment should mesh. “It’s a nice example of engineering on many levels, from integration of the components in a reactor all the way down to the mechanism at the atomistic level,” he said. “It fits with the themes of molecular nanotechnology, showing how we can scale it up to real-world devices.”
The next step in development of a scalable system is to improve upon the system’s stability and further reduce the amount of energy the process requires, Wang said.
Rice graduate students Peng Zhu and Chun-Yen Liu and Chuan Xia, the J. Evans Attwell-Welch Postdoctoral Fellow, are co-lead authors of the paper. Co-authors are Rice research scientist Guanhui Gao, postdoctoral researcher Xiao Zhang and graduate student Yang Xia; former Rice postdoctoral researcher Kun Jiang of Shanghai Jiao Tong University, China; and graduate student Yongjiu Lei and Husam Alshareef, a professor of material science and engineering, at King Abdullah University of Science and Technology, Saudi Arabia. Wang and Senftle are assistant professors of chemical and biomolecular engineering.
The NSF and the CIFAR Azrieli Global Scholars Program supported the research.
###
Read the abstract at https:/
This news release can be found online at https:/
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Water + air + electricity = hydrogen peroxide: http://news.
Rice reactor turns greenhouse gas into pure liquid fuel: http://news.
New catalyst turns pollutant into fuel: http://news.
Wang named to Forbes 30 Under 30 list: http://news.
The Wang Group: https:/
The Senftle Group: http://senftle.
Department of Chemical and Biomolecular Engineering: https:/
George R. Brown School of Engineering: https:/
Images for download:
https:/
An electron microscope images shows copper nanocubes used by Rice University engineers to catalyze the transformation of carbon monoxide into acetic acid. (Credit: Wang Group/Senftle Group/Rice University)
https:/
Rice University engineers have developed a reactor to produce liquid acetic acid directly from carbon monoxide. The reactor uses a catalyst of copper nanocubes and a solid-state electrolyte. (Credit: Illustration by Peng Zhu/Rice University)
https:/
CAPTION: Haotian Wang. (Credit: Rice University)
https:/
CAPTION: Thomas Senftle. (Credit: Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,978 undergraduates and 3,192 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Media Contact
Jeff Falk
[email protected]
Original Source
https:/
Related Journal Article
http://dx.