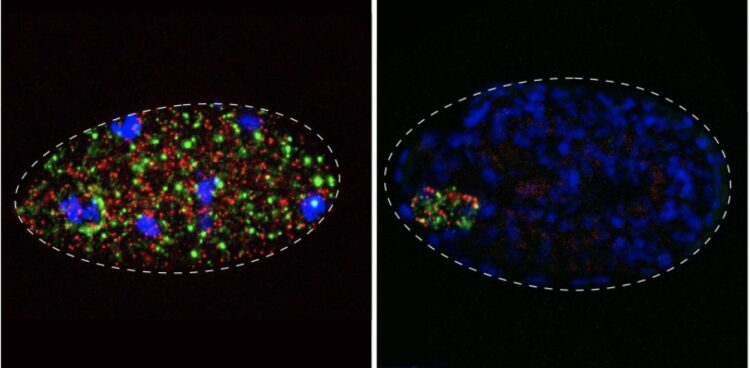
Credit: Photo credit: Pinelopi Pliota/IMBA
“Nature red in tooth and claw” – The battle to survive is fought down to the level of our genes. Toxin-antidote elements are gene pairs that spread in populations by killing non-carriers. Now, research by the Burga lab at IMBA and the Kruglyak lab at the University of California, Los Angeles shows that these elements are more common in nature than first thought and have evolved a wide range of mechanisms to force their inheritance and propagate in populations – a parasite within the genome.
Originally described in the model nematode Caenorhabditis elegans, toxin-antidote elements consist of two linked genes, a toxin and its antidote. While the toxin is loaded into eggs by mothers, only embryos that inherit the element express the antidote. Thus, the offspring must inherit the element to survive. In this way, toxin-antidote pairs promote their own survival and spread in the population. This comes at great expense of their host’s fitness – a quarter of their progeny, those that don’t inherit the element, fall prey to the toxin.
Arrested development
“Before our study, only a handful of toxin-antidote pairs were known, and they were serendipitously discovered in different labs. We wondered: what if we actually go and look for these selfish elements, would they be rare or common?”, says IMBA group leader Alejandro Burga. In their study, the teams of Burga and Kruglyak hunted for toxin-antidote pairs in a second nematode species Caenorhabditis tropicalis. The researchers identified five novel toxin-antidote pairs. Another selfish element was found in a third nematode species, C. briggsae. “This suggests that this class of selfish elements are not rare, but quite common in nematodes”, explains Burga. Surprisingly, some of the newly identified toxin-antidote pairs do not act by disrupting embryonic development – like those identified in C. elegans – but rather kill non-carriers at a later stage of development. One element, which Burga and his team genetically dissected, does not even go as far as killing non-carriers. Instead, this selfish element delays the onset of reproduction in non-carriers. This turns out to be enough to allow the element to spread through the population. The researchers are now further investigating its mechanism of action.
Selfishness may support diversity
Burga and colleagues made another startling observation. The researchers found that different toxin-antidote pairs can be located on homologous chromosomes. “Because of this pairing, not only do we see a conflict between the selfish element and the individual, but also between selfish elements, which try to kill each other”, says Burga. It is a war between selfish genes, but individuals get caught in the crossfire. “We hypothesize that selfish elements could have a direct role in speciation. At some point, carrying so many elements can just overwhelm their ability to produce viable offspring”, adds Eyal Ben-David, co-first author of the study, now Assistant Professor at the Hebrew University of Jerusalem. As a stark illustration of this, Burga and colleagues discovered that toxin-antidote elements can jointly cause defects in over 70% of the progeny from a single cross between two strains. “Such degree of incompatibility is likely insurmountable in the wild”, says Ben-David.
Knowledge gained from studying toxin-antidote pairs could be used to improve gene drive systems. Synthetic gene drives have been engineered in the lab to control vector-borne disease, for instance, by spreading genes that affect the fertility of mosquitos. However, gene drives are often hampered by mutations, and more resilient systems are needed to ensure success, says Burga. “By studying selfish elements, which are natural gene drive systems, we hope we can engineer better synthetic ones in the near future. Selfish elements have evolved over thousands of years in nematodes and spread around the world – nature indeed is the best teacher,” says Pinelopi Pliota, co-first author of the study.
Toxin-antidote pairs were first found in the 1980s in bacteria, and analogous elements have emerged in yeast, plants, insects and nematodes. “Toxin-antidote elements have independently evolved all over the tree of life. Either we humans are a unique case where they haven’t evolved, or we haven’t found them yet,” says Burga.
###
Original publication:
Eyal Ben-David et al.: “Ubiquitous selfish toxin-antidote elements in Caenorhabditis species”. Current Biology, 2021.
About IMBA
IMBA – Institute of Molecular Biotechnology – is one of the leading biomedical research institutes in Europe focusing on cutting-edge stem cell technologies, functional genomics, and RNA biology. IMBA is located at the Vienna BioCenter, the vibrant cluster of universities, research institutes and biotech companies in Austria. IMBA is a subsidiary of the Austrian Academy of Sciences, the leading national sponsor of non-university academic research. The stem cell and organoid research at IMBA is being funded by the Austrian Federal Ministry of Science and the City of Vienna.
Media Contact
Caterina Purini, MSc.
[email protected]