Credit: ©Science China Press
Highly efficient synthesis of ammonia under ambient temperatures and pressures has been drawing increasing attention for wide applications of scientists and industries around the world. Developing highly active and selective materials with transformative catalytic performance for ammonia synthesis is still a giant challenge.
Atomic catalysts (ACs) have long been the research frontier in the field of catalysis because of their unique structures and properties, such as the high atom utilization efficiency, high reaction selectivity and activity, etc. Compared with the traditional single-atom catalysts, graphdiyne (GDY)-based metal ACs feature unique and well-defined chemical and electronic structures, highly catalytic activity and selectivity, which is expected to achieve high selectivity and high yield of ammonia under ambient temperatures and pressures.
Recently, by utilizing the unique properties of GDY, Professor Yuliang Li (Academician of the Chinese Academy of Sciences) published a research paper in National Science Review (NSR), and proposed a new metal ion anchoring-electron transfer-self-reduction strategy for anchoring zero-valent palladium atoms. A free-standing 3D zero-valent atomic catalyst electrode was fabricated and showed high performance in electrocatalysis conversion of nitrogen to ammonia reaction at ambient conditions.
The scanning electron microscopy (SEM), high resolution transmission electron microscopy (TEM), aberration corrected high angle annular dark field (HAADF) scanning transmission electron microscopy (STEM), X-ray near-edge absorption structure (XANES) and extended X-ray absorption fine structure spectrum (EXAFS) results solidly demonstrated that the Pd atoms individually anchored on GDY and exhibited zero-valence state.
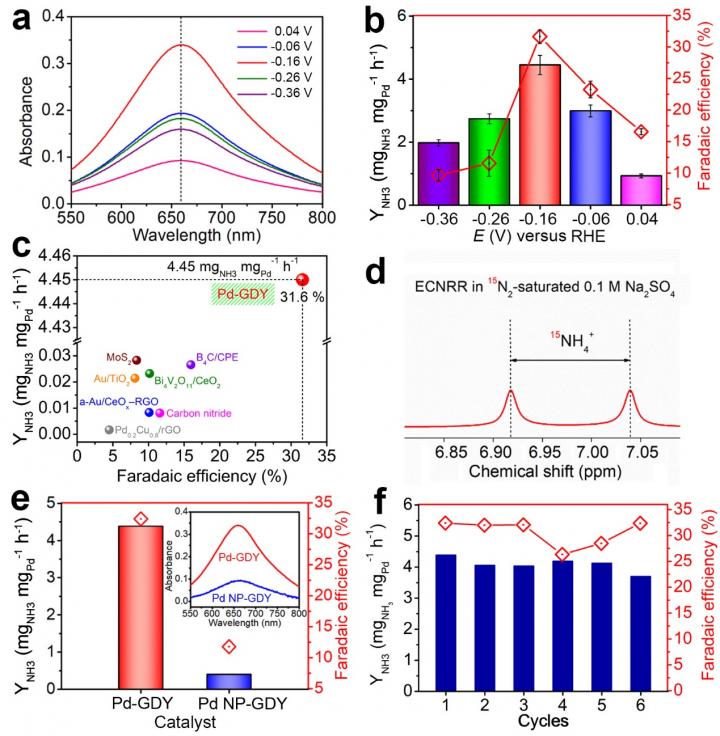
This catalyst shows unique advantages such as determined electronic and chemical structure, determined active sites, clear reaction mechanism and process, excellent properties and performance, etc. For example, in neutral conditions, the Pd-GDY catalyst has the highest ammonia yield of 4.45 ± 0.30 mgNH3 mgPd-1 h-1, which is nearly ten orders of magnitude higher than the reported materials; in acidic conditions, its catalytic activity can also reach up to 1.58 ± 0.05 mgNH3 mgPd-1 h-1. The 15N isotope labelling experiments confirmed that the NH3 was formed from the reduction of N2, revealing that the Pd-GDY is highly selective and active toward ammonia synthesis. Moreover, both the ammonia production rate and Faradaic efficiency of the catalyst can be maintained for several cycles without decay, confirming its robust stability.
###
See the article:
Graphdiyne Based Metal Atomic Catalysts for Synthesizing Ammonia
Huidi Yu, Yurui Xue, Lan Hui, Chao Zhang, Yan Fang, Yuxin Liu, Xi Chen, Danyan Zhang, Bolong Huang, Yuliang Li
Natl Sci Rev, 2020, doi: 10.1093/nsr/nwaa213
https:/
Media Contact
Yuliang Li
[email protected]
Related Journal Article
http://dx.




