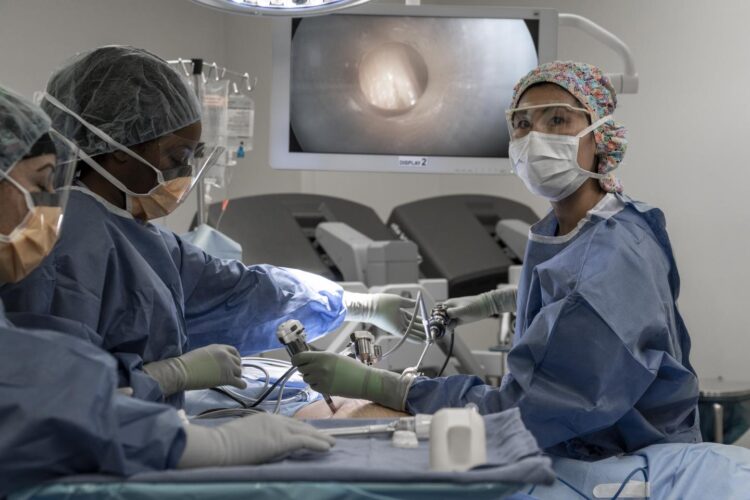
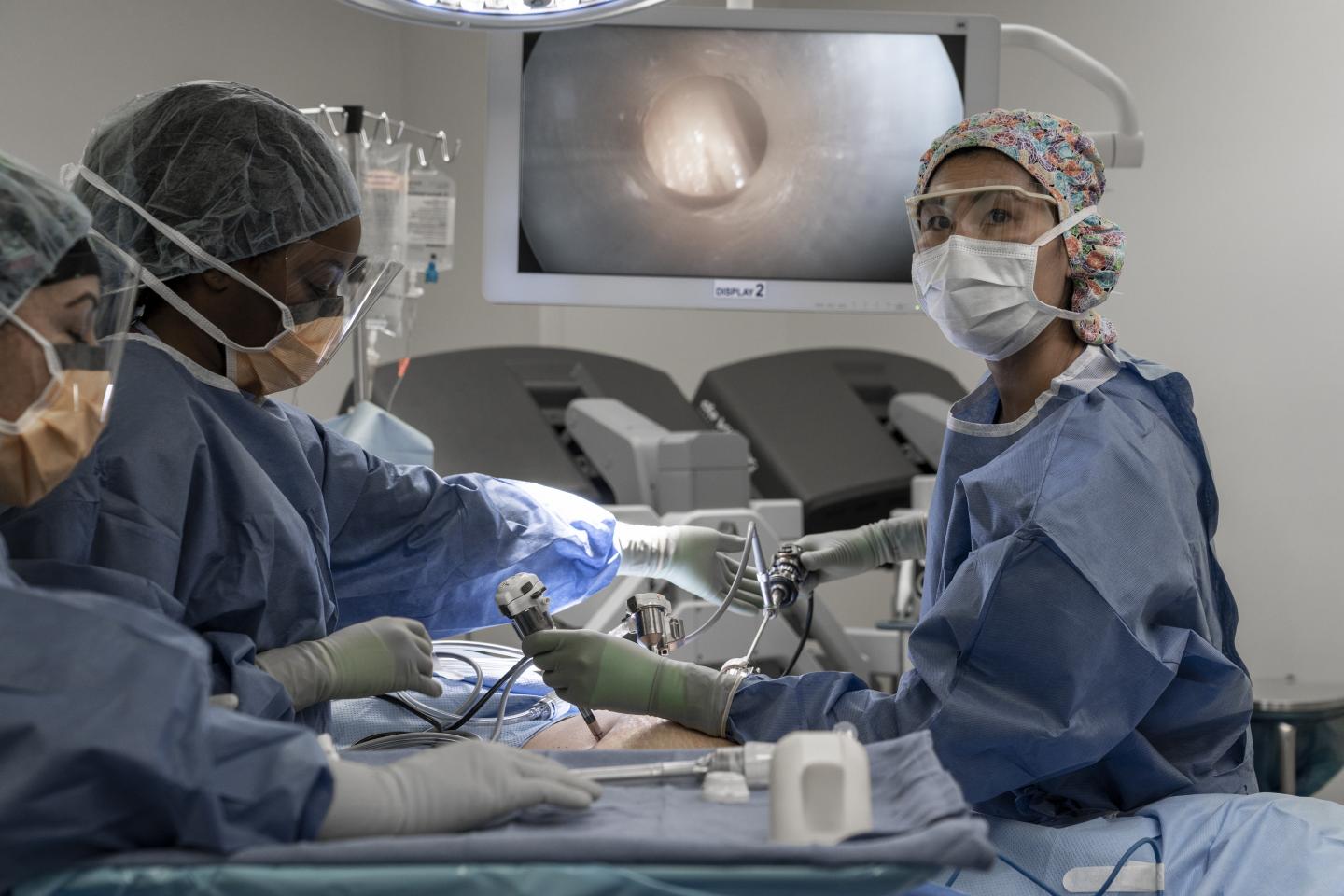
The experimental PIPAC treatment may extend the lives of people with advanced ovarian, uterine, gastric and colorectal cancers whose current options are only local disease control and palliative care.
Credit: City of Hope
DUARTE, Calif. — City of Hope surgical researchers are the first in the nation to open a clinical trial that could one day become an effective way to deliver chemotherapy to abdominal cancer patients who currently have very few treatment options.
Ovarian, uterine, gastric and colorectal cancers can be difficult to treat using traditional intravenous chemotherapy, especially when the tumors spread to a thin layer of tissue that lines the abdomen called the peritoneum. Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, said Thanh Dellinger, M.D., gynecologic oncology surgeon at City of Hope and principal investigator of the multisite phase 1 clinical trial of pressurized intraperitoneal aerosolized chemotherapy (PIPAC).
“City of Hope has a long history as a leader in delivering intraperitoneal chemotherapy and performs a majority of what some might consider the first iteration of PIPAC: HIPEC, a heated chemotherapy procedure that requires surgical removal of tumors prior to flooding the abdominal cavity with scalding chemotherapy,” Dellinger said. “Certain abdominal cancer patients whose disease cannot be surgically removed or who might not be able to survive major surgery could now undergo a short, laparoscopic surgery and then go home to recover within a day or two.”
City of Hope performs the most HIPEC (heated intraoperative peritoneal chemotherapy) procedures among medical centers in Los Angeles County and is one of the few in the county to offer HIPEC for ovarian cancer.
For PIPAC, two small incisions are made while the patient is under general anesthesia. Using narrow tubes called trochars, experienced surgeons blow the abdomen up like a balloon to provide a clear path for the body to receive therapy. Safely within these attached tubes, liquid chemotherapy is transformed into a fine spray that reaches all the crevices of the abdomen. After 30 minutes of soaking in this mist bath, the chemotherapy droplets are vacuumed out and properly placed into cytotoxic waste bins.
The pressurized chemotherapy spray seeps deeper into tissue. Because of this effective technique, only 10-20% of the usual chemotherapy dose is required, which lowers the treatment’s toxicity, making it more tolerable for patients who have to manage side effects. Notably, patients do not experience hair loss when chemotherapy is delivered via PIPAC, Dellinger said.
Many gastric and colorectal cancer patients who could qualify for this clinical trial have limited quality of life due to their current treatments, said Mustafa Raoof, M.D., M.S., surgical oncologist at City of Hope and co-principal investigator of the clinical trial.
“They may need to go to the hospital every week to undergo painful drainage of the abdomen, or they may have to live life attached to a bag that holds their urine or feces,” Raoof said. “We want our patients to be able to live their best life, and PIPAC may enable them to do exactly that.”
The researchers will evaluate the safety and efficacy of PIPAC in ovarian, uterine, colorectal and gastric cancer patients with peritoneal carcinomas. Under normal circumstances, these patients are expected to have a life expectancy of less than six months.
Patients who have failed at least one previous standard chemotherapeutic treatment may qualify and be enrolled in one of two arms of the study, which will use varying doses of either cisplatin and doxorubicin or oxaliplatin preceded by fluorouracil and leucovorin. The participants will receive these treatments every six weeks for a total of three cycles. The trial is expected to last three years and have a maximum of 24 patient participants. The other trial sites will be Mayo Clinic in Florida, Northwell Health in New York and the National Cancer Institute in Maryland.
City of Hope researchers will also perform additional diagnostic laparoscopy to obtain tumor tissue for molecular testing of each patient’s entire protein-coding DNA and RNA. The objective is to see if they are eligible for other clinical trials and novel biological therapies. This is part of City of Hope’s precision medicine strategy — to understand each patient’s specific disease to be able to offer the most leading-edge, personalized treatment options.
“Although not part of this clinical trial, we hope to extend the study by taking a biopsy of the tumor, creating an avatar of it in the lab, and testing different or new drug combinations to see what works best,” Raoof said. “In the future, we may be able to use PIPAC to deliver novel therapeutics developed at City of Hope. Because PIPAC requires a lower systemic dose, the treatment theoretically will be less toxic than today’s standard-of-care treatments.”
For more information on the clinical trial, please go to rb.gy/qrf02n.
###
About City of Hope
City of Hope is an independent biomedical research and treatment center for cancer, diabetes and other life-threatening diseases. Founded in 1913, City of Hope is a leader in bone marrow transplantation and immunotherapy such as CAR T cell therapy. City of Hope’s translational research and personalized treatment protocols advance care throughout the world. Human synthetic insulin and numerous breakthrough cancer drugs are based on technology developed at the institution. A National Cancer Institute-designated comprehensive cancer center and a founding member of the National Comprehensive Cancer Network, City of Hope is the highest ranked cancer hospital in the West, according to U.S. News & World Report’s Best Hospitals: Specialty Ranking. Its main campus is located near Los Angeles, with additional locations throughout Southern California. For more information about City of Hope, follow us on , , YouTube or Instagram.
Media Contact
Zen Vuong
[email protected]