Credit: Kobe University
A large-scale analysis of the clinical characteristics of Alport syndrome in Japanese patients has revealed that the effectiveness of existing treatment with ACE inhibitors and/or angiotensin receptor blockers (RAS inhibitors) (*1) varies depending on the type of mutation in the syndrome’s causal gene (COL4A5). RAS inhibitors are widely administered to patients with chronic kidney diseases as they are known to preserve kidney functions, and they are also effective against Alport Syndrome. In addition to providing proof of the effectiveness of RAS inhibitor treatment in Japanese patients with Alport syndrome, the researchers revealed for the first time in the world that the degree of effectiveness depends on the genotype.
The research group included Assistant Professor YAMAMURA Tomohiko, Professor IIJIMA Kazumoto and Professor NOZU Kandai et al. of the Department of Pediatrics, Kobe University Graduate School of Medicine.
These results were published online in the journal ‘Kidney International‘ on July 23, 2020.
Main Points
- Alport syndrome is a hereditary disorder characterized by nephritis, hearing loss and eye abnormalities (such as anterior lenticonus or cataracts).
- X-linked (*2) Alport syndrome is the most common form and men in particular exhibit severe symptoms. Those who have a severe mutation (such as a nonsense mutation (*3)) in Alport syndrome’s casual gene COL4A5 are known to develop end-stage kidney disease (ESKD) in their early twenties.
- One promising treatment involves using ACE inhibitors and/or angiotensin receptor blockers (RAS inhibitors) to preserve kidney functions. So far, studies conducted outside Japan have shown these treatments to be effective for lowering urinary protein levels and suppressing the progression of kidney function deterioration.
- This research team conducted a large scale survey of Japanese male patients with Alport syndrome. They compared the median results of a group of patients who were prescribed RAS inhibitors with a group of patients who didn’t take the drug. They found that the age at which patients taking RAS inhibitors progressed to ESKD was delayed by over 20 years, demonstrating the effectiveness of this treatment.
- The team also compared the effectiveness of the treatment for those with minor mutations (such as missense mutations (*4)) and those with severe mutations in the casual gene. Although RAS inhibitors were effective in treating those with severe mutations, they were comparatively more effective in those with minor mutations.
- For the first time in the world, this research demonstrated that the effectiveness of RAS inhibitors differs depending on the genotype of Alport syndrome.
Research Background
Alport syndrome (AS) is the second most commonly occurring hereditary kidney disease after autosomal dominant polycystic kidney disease (ADPKD). There is one case of Alport syndrome in every 5,000 to 10,000 people. It is characterized by hearing loss, eye abnormalities and kidney disease, often progressing to end stage kidney disease (ESKD). Alport syndrome is divided into three groups according to how it is inherited; X-linked AS, autosomal recessive AS and autosomal dominant AS with approximately 80% of cases being X-linked Alport syndrome (XLAS).
XLAS develops due to pathological mutations in the COL4A5 gene that encodes the type IV collagen α5 chain. In particular, male patients with XLAS are likely to have severe symptoms and around 90% of them experience ESKD by the age of 40. This requires them to undergo renal replacement therapies such as dialysis and kidney transplants. However, there is still no specialized treatment for Alport syndrome itself.
Men with severe types of Alport syndrome (such as those caused by nonsense mutations in the COL4A5 gene) develop ESKD over ten years earlier than those with milder forms (such as those caused by missense mutations). A possible treatment for Alport syndrome involves using ACE inhibitors and/or angiotensin receptor blockers (RAS inhibitors) to preserve kidney functions. So far, studies conducted outside Japan have shown these treatments to be effective for lowering urinary protein levels and suppressing the progression of kidney function deterioration.
Research Findings
Up until now, this research team has established a comprehensive genetic diagnosis system for Alport syndrome, which has enabled them to conduct genetic diagnoses of Japanese patients. This time, the researchers conducted a retrospective investigation into the clinical characteristics of the disorder in 430 male patients with XLAS. From these results and by drawing on the findings of other research, they revealed the following:
1. It was possible to analyze the clinical data of 422 of these patients, and the results showed that the median age for progression to ESKD was 35.
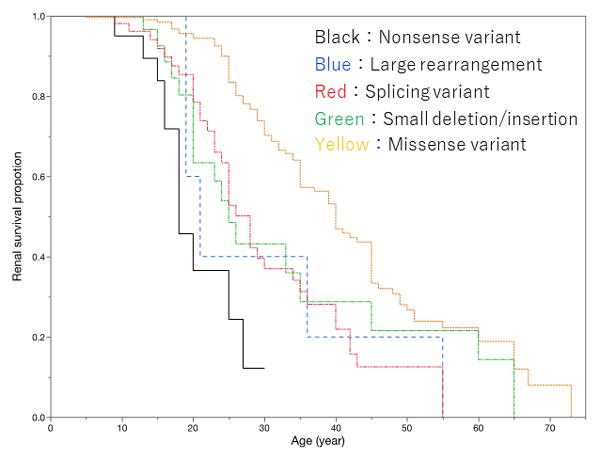
2. There was a very strong correlational relationship between genotype and the median age for progression to ESKD. The median age was 18 for those with nonsense mutations (Figure 1: black survival curve), whereas it was 40 in those with missense mutations (Figure 1: yellow survival curve). This is a difference of 22 years (Figure 1).
3. The data also revealed a connection between the symptom of hearing loss and the median age at which ESKD developed. The median age in patients with hearing loss was 28, in those without hearing loss it was 55. This showed that renal symptoms were more severe in cases with hearing loss.
4. Clinical data from 207 patients that showed whether or not they were prescribed RAS inhibitors was also analyzed. The results revealed that those who didn’t receive RAS inhibitor treatment developed ESKD by a median age of 28, whereas over half of those prescribed with RAS inhibitors did not develop ESKD before the age of 50. In other words, it was shown that this drug could delay the onset of ESKD by over 20 years (Figure 2).
5. Subsequently, the researchers evaluated the effectiveness of RAS inhibitors depending on whether the patient had a severe mutation or a minor mutation. In patients with minor mutations, those who were not administered RAS inhibitors developed ESKD at a median age of 33 (Figure 3: red survival curve), whereas over half of those who received treatment with the drug did not develop ESKD before a median age of 50 (Figure 3: blue survival curve).
On the other hand, in patients with severe mutations, the group who weren’t prescribed RAS inhibitors progressed to ESKD by a median of 16 years of age (Figure 3: black survival curve), whereas those receiving RAS inhibitor treatment developed ESKD at a median age of 28 (Figure 3: green survival curve). Therefore, the drug was effective in treating Alport syndrome caused by severe mutations as it delayed the onset of ESKD in patients by a median of 12 years. However the treatment was shown to be less effective than in patients with minor mutations (Figure 3).
###
Conclusion
RAS inhibitors were effective, even for patients with severe mutations, however the median age for progression to end stage renal failure for those with severe forms of the syndrome was 28. The current team is currently developing a treatment method (Exon skipping therapy (*5)) for patients with severe mutations, and the above results are likely to increase the demand for this treatment’s development.
Reference: New treatment method for Alport Syndrome uses antisense oligonucleotides: https:/
Glossary
1. ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers (ARB) (RAS inhibitors):
The Renin-Angiotensin-Aldosterone System (RAS, or RAAS) is responsible for regulating blood pressure. RAS inhibitors have a hypotensive effect as they block a number of hormones that raise blood pressure. Originally prescribed as a blood pressure lowering medication, in recent years these inhibitors have been shown to be effective against chronic kidney diseases, such as diabetic nephropathy, as they preserve kidney functions.
2. X-linked form of a disease:
A hereditary disease that occurs as a result of a mutation in a gene that coded the X chromosome. The symptoms are more severe in men because they only have one X chromosome, whereas women have two.
3. Nonsense mutation:
A nonsense mutation is where the substitution of a single base pair leads to the premature appearance of a stop codon. This causes the formation of proteins at the mutation site to be stopped halfway, resulting in the production of shortened and usually defective proteins. On the other hand, if a missense mutation occurs at the same mutation site, this causes a different amino acid to be incorporated into the protein, however the proteins are fully developed. This is why the most severe form of XLAS is caused by nonsense mutations.
4. Missense mutation:
A missense mutation is where a single amino acid of a single base pair is replaced by a different amino acid. This is different to a nonsense mutation because complete proteins are generated. Consequently, in genetic disorders, patients with missense mutations in the causal gene have minor clinical manifestations compared to those with nonsense mutations. In male patients with X-linked Alport syndrome, this link between genotype and clinical manifestations is well recognized.
5.Exon skipping therapy:
A treatment method that can change severe mutations into minor mutations by using a nucleic acid medicine to skip over exons with nonsense mutations. So far, this treatment has been shown to be effective in research on animals (Yamamura T et al, Nature Communications 2020).
Acknowledgements
From April 2017 onwards, this research has been continued based on funding from the Japan Agency for Medical Research and Development (AMED) ‘Step 0’ (which supports exploratory research into revolutionary treatments and medical devices for intractable and rare diseases).
Journal Information
Title:
Genotype-phenotype correlation and the influence of the genotype on response to angiotensin-targeting drugs in Japanese patients with male X-linked Alport syndrome
DOI?10.1016/j.kint.2020.06.038
Authors:
Tomohiko Yamamura1, Tomoko Horinouchi1, China Nagano1, Takashi Omori2, Nana Sakakibara1, Yuya Aoto1, Shinya Ishiko1, Koichi Nakanishi3, Yuko Shima4, Hiroaki Nagase1, Hiroki Takeda1, Rini Rossanti1, Ming Juan Ye1, Yoshimi Nozu1, Shingo Ishimori1, Takeshi Ninchoji1, Hiroshi Kaito1, Naoya Morisada1, Kazumoto Iijima1, Kandai Nozu1
1 Department of Pediatrics, Kobe University Graduate School of Medicine
2 Clinical and Translational Research Center, Kobe University Hospital
3 Department of Child Health and Welfare (Pediatrics), Graduate School of Medicine, University of the Ryukyus
4 Department of Pediatrics, Wakayama Medical University
Journal:
Kidney International
Media Contact
Verity Townsend
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.




