Credit: Tokyo Tech
Many reactions that we use to produce chemical compounds in food, medical, and industrial fields would not be feasible without the use of catalysts. A catalyst is a substance that, even in small quantities, accelerates the rate of a chemical reaction and sometimes allows it to occur at milder conditions (lower temperature and pressure). A good catalyst can sometimes multiply the throughput of an industrial-scale reactor or shave more than 100°C off of its operating temperature.
It is no surprise, then, that catalyst research is crucial for making chemical reactions more efficient. One emerging approach that has been observed to provide these benefits is heating the metal nanoparticles in some catalysts directly using microwaves instead of conventional uniform heating techniques. Metal nanoparticles in catalysts interact strongly with microwaves and are believed to be heated selectively. However, scientists have reported conflicting results when using this approach, and understanding the effect that selectively heating the nanoparticles has on chemical reactions is difficult because no methods for measuring their local temperature have been found yet.
Now, scientists at Tokyo Tech led by Prof Yuji Wada tackle this problem and demonstrate a novel approach for measuring the local temperature of platinum nanoparticles in a solid catalyst. Their method, as detailed in their study published in Communications Chemistry, relies on X-ray absorption fine structure (XAFS) spectroscopy, which, as the name implies, provides information on the small local structures of a material using X-rays.
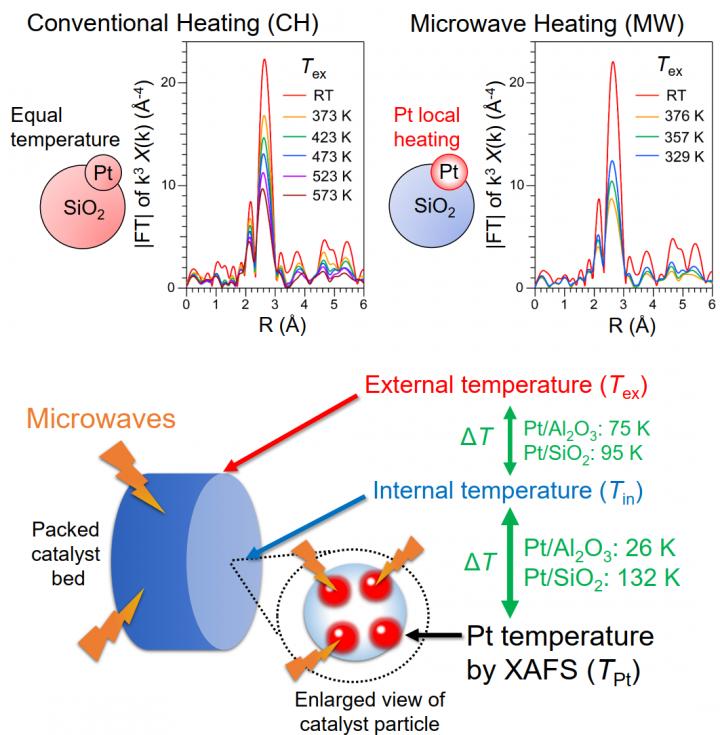
In extended XAFS oscillations, a value called the Debye-Waller factor can be derived. This factor is comprised of two terms; one related to structural disorder, and one related to thermal disorder. If the structure of the catalyst does not change upon microwave heating, any variation in the Debye-Waller factor has to be due to thermal variations. Therefore, XAFS can be used to indirectly measure the temperature of metal nanoparticles, as shown in Figure 1.
The team of scientists tested this approach in “platinum on alumina” and “platinum on silica” catalysts to find out to what extent microwaves can selectively heat the platinum nanoparticles instead of their supporting material. Microwave heating was found to produce a marked temperature difference between NP and support. A series of comparative experiments demonstrated that a higher local temperature of the metal nanoparticles in catalysts is crucial to obtaining higher reaction rates at the same temperature.
Excited about the results, Prof Wada remarks: “This work is the first to present a method for the assessment of the local temperatures of nanoparticles and their effect on catalytic reactions. We conclude that the local heating of platinum nanoparticles is efficient for accelerating chemical reactions that involve platinum itself, presenting a practical approach to obtain a dramatic enhancement in catalytic reactions using microwave heating.”
These findings represent a breakthrough for improving our understanding of the role of microwave heating in enhancing catalytic performance. Dr. Tsubaki adds, “Efficient energy concentration at the active sites of catalysts–the metal nanoparticles in this case–should become a critical strategy for exploring microwave chemistry to achieve efficient energy use for reactions and to enable milder conditions for reaction acceleration.” This new insight into catalytic processes will hopefully save tons of energy in the long run by making reactors work smarter, not harder.
###
Media Contact
Kazuhide Hasegawa
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




