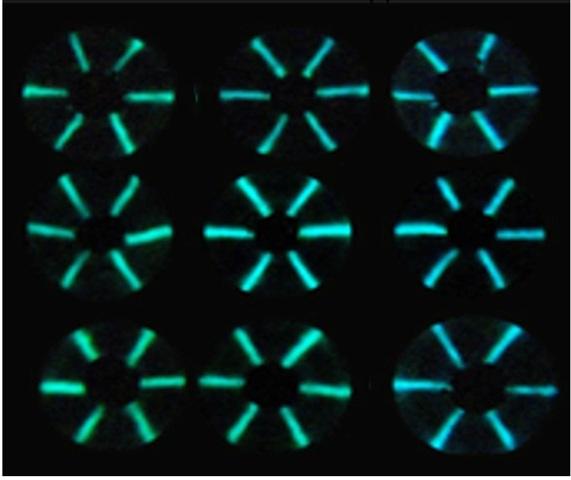
Credit: Adapted from ACS Sensors 2020, DOI: 10.1021/acssensors.0c00564
To defend the body, the immune system makes proteins known as antibodies that latch onto the perceived threat, be it HIV, the new coronavirus or, as is the case in autoimmune disease, part of the body itself. In a new proof-of-concept study in ACS Sensors, researchers describe a new system for detecting antibodies within a pinprick of blood within minutes, using an unlikely combination of cotton thread, glowing proteins and a smartphone camera.
While some tests simply detect the presence of an antibody, sometimes doctors want to know how much is circulating in the blood. Such quantitative tests are used to diagnose a number of conditions, including infections and autoimmune diseases. Although a quantitative antibody test is not yet approved for use in the U.S., such a test could potentially aid in assessing immunity to SARS-CoV-2. However, quantitative testing currently requires expensive, sophisticated instruments in labs, and efforts to make it more accessible have had only limited success. So, Maarten Merkx, Daniel Citterio and colleagues tested an approach that could provide a small, inexpensive alternative.
The researchers’ microfluidic thread-based analytical device (μTAD) relies on light-emitting sensor proteins held on a thread. In the presence of the right antibodies, the color of the light emitted by the sensors changes. The shift, from green to blue, correlates with the concentration of antibodies in a sample. Using a finger-prick-sized drop of pigs’ blood spiked with antibodies against HIV, the team showed that their system could successfully detect antibody levels within five minutes. In addition, the device can test for the amounts of several different antibodies in a single blood sample and doesn’t require extensive handling and incubation steps. They found that a smartphone camera, outfitted with an adaptor, could pick up on the shifts in the light’s color, while the device itself could convert color data into test results and transmit that information. With further development, this combination of technologies could provide user-friendly, one-step analysis of antibody concentration, according to the researchers.
###
The authors acknowledge funding from the Japan Society for the Promotion of Science through a Bilateral Joint Research Projects/Seminars grant and a Grant-in-aid for Scientific Research, the Terumo Life Science Foundation, and the European Research Council Starting and Proof of Concept grants.
The abstract that accompanies this paper can be viewed here.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and its people. The Society is a global leader in providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a specialist in scientific information solutions (including SciFinder® and STN®), its CAS division powers global research, discovery and innovation. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact [email protected].
Follow us: Twitter | Facebook
Media Contact
Katie Cottingham
[email protected]




