Credit: College of Engineering, Carnegie Mellon University
Researchers at Carnegie Mellon University have developed a method for self-assembling nanostructures with gamma-modified peptide nucleic acid (γPNA), a synthetic mimic of DNA. The process has the potential to impact nanomanufacturing as well as future biomedical technologies like targeted diagnostics and drug delivery.
Published this week in Nature Communications, the work introduces a science of γPNA nanotechnology that enables self-assembly in organic solvent solutions, the harsh environments used in peptide and polymer synthesis. This holds promise for nanofabrication and nanosensing.
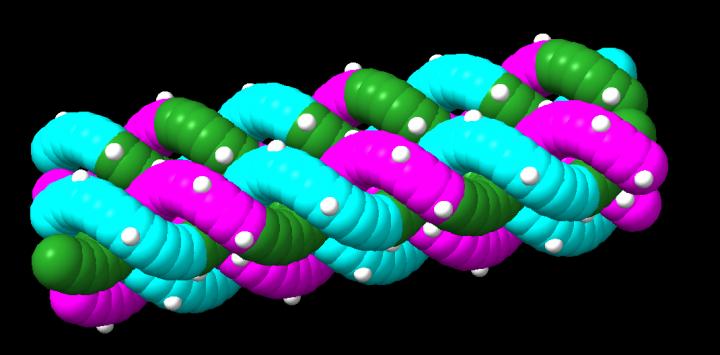
The research team, led by Assistant Professor of Mechanical Engineering Rebecca Taylor, reported that γPNA can form nanofibers in organic solvent solutions that can grow up to 11 microns in length (more than 1000 times longer than their width). These represent the first complex, all-PNA nanostructures to be formed in organic solvents.
Taylor, who heads the heads the Microsystems and MechanoBiology Lab at Carnegie Mellon, wants to leverage PNA’s “superpowers.” In addition to its higher thermal stability, γPNA retains the ability to bind to other nucleic acids in organic solvent mixtures that would typically destabilize structural DNA nanotechnology. This means that they can form nanostructures in solvent environments that prevent formation of DNA-based nanostructures.
Another property of γPNA is that it is less twisted than the double helix of DNA. The result of this difference is that the “rules” for designing PNA-based nanostructures are different than the rules for designing structural DNA nanotechnology.
“As mechanical engineers, we were prepared for the challenge of solving a structural design problem, Taylor said. “Due to the unusual helical twist, we had to come up with a new approach for weaving these pieces together.”
Because the researchers in Taylor’s lab seek to use dynamic shape change in their nanostructures, they were intrigued to discover that morphological changes – like stiffening or unraveling – occurred when they incorporated DNA into the γPNA nanostructures.
Other interesting characteristics that the researchers want to explore further include solubility in water and aggregation. In water, these current nanofibers tend to clump together. In organic solvent mixtures, the Taylor lab has demonstrated that they can control whether or not structures aggregate, and Taylor believes that the aggregation is a feature that can be leveraged.
“These nanofibers follow the Watson-Crick binding rules of DNA, but they appear to act more and more like peptides and proteins as PNA structures grow in size and complexity. DNA structures repel each other, but these new materials do not, and potentially we can leverage this for creating responsive surface coatings,” said Taylor.
The synthetic γPNA molecule has been perceived as a simple DNA mimic having desirable properties such as high biostability and strong affinity for complementary nucleic acids.
“We believe through this work, we could additionally adjust this perception by highlighting the ability of γPNA to act as both – as a peptide mimic because of its pseudopeptide backbone and as a DNA mimic because of its sequence complementarity. This change in perception could allow us to understand the multiple identities this molecule can leverage in the world of PNA nanostructure design,” said Sriram Kumar, a mechanical engineering Ph.D. candidate and the first author on the paper.
Although PNA is already being used in groundbreaking gene therapy applications, there is still a lot to learn about this synthetic material’s potential. If complex PNA nanostructures can someday be formed in aqueous solutions, Taylor’s team hopes that additional applications will include enzyme-resistant nanomachines including biosensors, diagnostics, and nanorobots.
“PNA-peptide hybrids will create a whole new toolkit for scientists,” Taylor said.
The researchers used custom gamma modifications to PNA that were developed by Danith Ly’s lab at Carnegie Mellon. Future work will investigate left-handed γPNAs in the nanomanufacturing process. For future biomedical applications, left-handed structures would be of particular interest because they would not pose a risk of binding to cellular DNA.
###
This work represents an interdisciplinary collaboration. Additional authors included chemistry Ph.D. candidate Alexander Pearse and mechanical engineering candidate Ying Liu.
Media Contact
Lisa Kulick
[email protected]
Related Journal Article
http://dx.




