Experiments find the sweet spot for surface area and bubbles to store energy
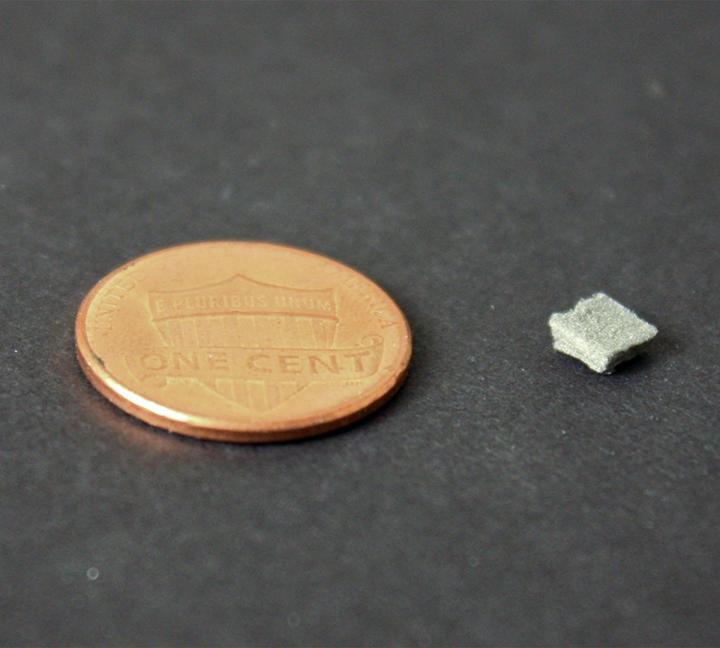
Credit: Wiley Lab, Duke University
DURHAM, N.C. — Electrolysis, passing a current through water to break it into gaseous hydrogen and oxygen, could be a handy way to store excess energy from wind or solar power. The hydrogen can be stored and used as fuel later, when the sun is down or the winds are calm.
Unfortunately, without some kind of affordable energy storage like this, billions of watts of renewable energy are wasted each year.
For hydrogen to be the solution to the storage problem, water-splitting electrolysis would have to be much more affordable and efficient, said Ben Wiley, a professor of chemistry at Duke University. And he and his team have some ideas about how to accomplish that.
Wiley and his lab recently tested three new materials that might be used as a porous, flow-through electrode to improve the efficiency of electrolysis. Their goal was to increase the surface area of the electrode for reactions, while avoiding trapping the gas bubbles that are produced.
“The maximum rate at which hydrogen is produced is limited by the bubbles blocking the electrode – literally blocking the water from getting to the surface and splitting,” Wiley said.
In a paper appearing May 25 in Advanced Energy Materials, they compared three different configurations of a porous electrode through which the alkaline water can flow as the reaction occurs.
They fabricated three kinds of flow-through electrodes, each a 4 millimeter square of sponge-like material, just a millimeter thick. One was made of a nickel foam, one was a ‘felt’ made of nickel microfibers, and the third was a felt made of nickel-copper nanowires.
Pulsing current through the electrodes for five minutes on, five minutes off, they found that the felt made of nickel-copper nanowires initially produced hydrogen more efficiently because it had a greater surface area than the other two materials. But within 30 seconds, its efficiency plunged because the material got clogged with bubbles.
The nickel foam electrode was best at letting the bubbles escape, but it had a significantly lower surface area than the other two electrodes, making it less productive.
The sweet spot turned out to be a felt of nickel microfiber that produced more hydrogen than the nanowire felt, despite having 25 percent less surface area for the reaction.
Over the course of a 100-hour test, the microfiber felt produced hydrogen at a current density of 25,000 milliamps per square centimeter. At that rate, it would be 50 times more productive than the conventional alkaline electrolyzers currently in use for water electrolysis, the researchers calculated.
The cheapest way to make industrial quantities of hydrogen right now isn’t by splitting water, but by breaking natural gas (methane) apart with very hot steam – an energy-intensive approach that creates 9 to 12 tons of C02 for every ton of hydrogen it yields, not including the energy needed to create 1000-degree Celsius steam.
Wiley said commercial producers of water electrolyzers may be able to make improvements in the structure of their electrodes based on what his team has learned. If they could greatly increase the hydrogen production rate, the cost of hydrogen produced from splitting water could go down, perhaps even enough to make it an affordable storage solution for renewable energy.
He is also working with a group of students in Duke’s Bass Connections program who are exploring whether flow-through electrolysis might be scaled up to make hydrogen from India’s abundant solar power.
###
This research was supported by National Science Foundation CAREER award (DMR- 1253534), and a grant from the Duke University Energy Initiative.
CITATION: “Alkaline Water Electrolysis at 25 A cm-2 with a Microfibrous Flow-through Electrode,” Feichen Yang, Myung Jun Kim, Micah Brown, and Benjamin J. Wiley. Advanced Energy Materials, May 25, 2020. DOI: 10.1002/aenm.202001174
Media Contact
Karl Leif Bates
[email protected]
Related Journal Article
http://dx.




