Researchers at UNIGE have developed a new method for creating chains of molecular rings with unparalleled sophistication.
Credit: © UNIGE
Cyclic molecules are everywhere, and everything around us stems from the way they are assembled: not just taste, colour and smell but also (for example) pharmaceutical drugs. Nature by itself forms molecular rings of different sizes and chains of rings of varying lengths that scientists are able to reproduce artificially. Chemists from the University of Geneva (UNIGE) have now devised a new technique for creating these chains of molecular rings that do not use standard chemical interactions but contact with large molecular surfaces that are electron-poor and do not exist in nature. Unlike with standard procedures, this new technique works by autocatalysis – the rarest, but also the most ambitious, type of transformation that exists in chemistry. The results of this research, published in the journal Angewandte Chemie, open up new prospects for molecular cyclization and also provide the first part of the answer to an old contradiction in classical chemistry.
The molecules that surround us are often arranged in the shape of cycles, forming steroids, sugars, perfumes or also drugs, for example. In organic chemistry, these molecular rings can be created using the technique of catalysis: the selected molecule, called a substrate, is placed in contact with the molecule that realizes the transformation – the catalyst – usually through hydrogen bonds. But with this single method of interaction, the creative possibilities are reduced. Incorporating new ways of interaction would convert them differently, thereby creating new materials with the potential to solve scientific and societal problems that are intractable with conventional methods.
Stefan Matile is a professor in the Department of Organic Chemistry in the School of Chemistry and Biochemistry of UNIGE’s Faculty of Sciences. He is also a member of the NCCR Chemical Biology and the NCCR Molecular Systems Engineering. “Our laboratory has specialised in implementing new contacts between molecules, one of them based on very large molecular surfaces, known as aromatics, which are poor in highly-delocalised electrons.” Professor Matile adds that contacts with these large, empty molecular plains, which are absent in nature, seemed promising for the cyclization of molecular rings that are chained to each other. But what are the consequences?
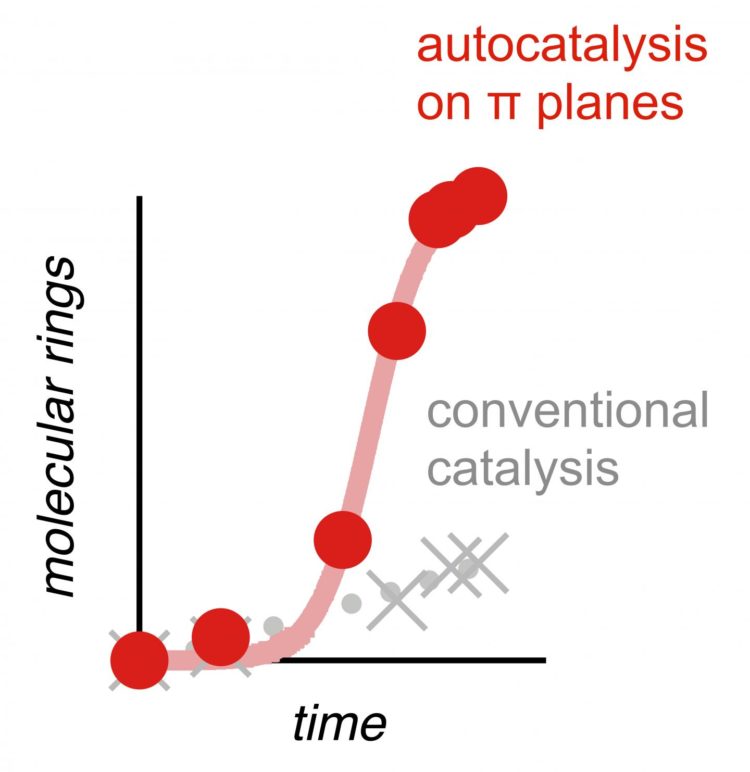
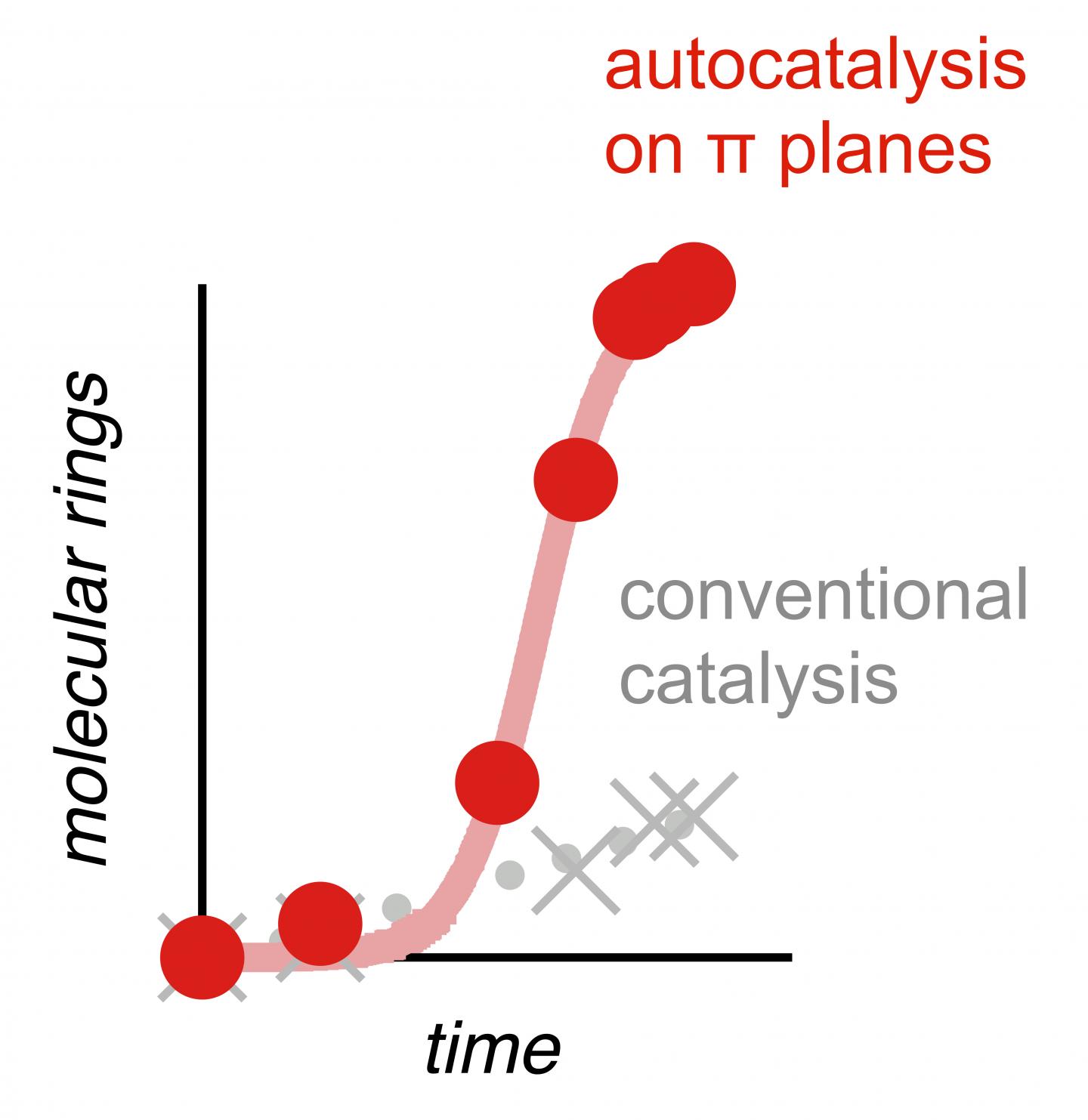
Chains of molecular rings produced by autocatalysis
The aims of the Geneva chemists were: to reproduce cycles of different sizes, i.e. consisting of a number of defined atoms (steroids, for instance, are formed from three cycles of six atoms plus one of five); and to link several cycles together without using the hydrogen bonds but a molecular surface low in delocalised electrons (known as anion-π interactions). “The main characteristic of this molecular plain is the empty space it provides for molecules to assemble”, says Miguel Paraja, a researcher in UNIGE’s Department of Organic Chemistry. On contact with this new, spacious and electron-deficient surface, the molecules formed cycles of different sizes (4 to 8 atoms) and various sequences. “But the big news was the way the transformations occurred!” adds the Geneva-based chemist.
All these cyclizations took place autocatalytically. “With a conventional catalyst, the cyclizations are fast at the start, and then – since there is less and less substrate – they increasingly slow down, explains Xiaoyu Hao, a researcher in the same laboratory. But with autocatalysis, it’s the very opposite that happens!” Indeed, the molecular transformations accelerate on a massive scale. “Although this autocatalysis is a very rare transformation phenomenon in chemistry, it is also the most astonishing, says professor Matile. “It’s based on mutual aid between molecules: the first molecules transformed help the next to transform, which isn’t the case during normal catalysis, which decelerates rather than accelerates.”
The first step in answering an old contradiction of classical chemistry
This discovery helps answer one of the oldest contradictions in classical chemistry. “There is a very well-known chain of molecular rings, called a brevetoxin, which is found in the red tide and which has the effect of killing fish”, explains professor Matile. It was discovered by a towering figure in organic chemistry, Koji Nakanishi, who put forward an explanation for the possible construction of this extraordinary chain formed from eleven consecutive molecular rings in a single reaction. But this hypothesis did not agree with Jack Baldwin, a famous chemist who produced the rules explaining the formation of cycles that are now accepted as the basis of classical chemistry. The “Nakanishi hypothesis” violates these rules for every of the eleven rings. “Our rings can be formed according to Baldwin’s rules if we want them to, reports Paraja. More importantly, we can also break the Baldwin rules on demand with our new catalysts and create those forbidden rings that Koji Nakanishi dreamed of.” “The key to success, explains Hao, is the large empty space offered by our new catalysts.”
Professor Matile continues that: “With the discovery of autocatalysis in forming cyclic molecules, our anion-π contacts have helped us understand the most subtle way to transform the molecules that exists in chemistry. And this will help us create new chains of molecular rings.” The chemists will be able to influence and direct the nature of the transformation of the next substrate, creating new materials, one of the main objectives also of the NCCR Molecular Systems Engineering. “Most solutions to scientific problems, be they about food, medicine or environment, involve molecules and new contacts that can be created among them”, says the Geneva-based chemist.
###
Media Contact
Stefan Matile
[email protected]
41-223-796-523
Related Journal Article
http://dx.