Credit: ©Science China Press
“Rock-chair” Li-ion battery (LIB) was discovered in the late 1970s and commercialized in 1991 by Sony, which has become the priority way we store portable energy today. To honor the contribution for “creating a rechargeable world”, the 2019 Nobel Prize in chemistry was awarded to three famous scientists (John B. Goodenough, M. Stanley Whittingham, Akira Yoshino) who made the most important contributions to the discovery of LIBs. However, this technology is nearing its practical performance limits and extensive efforts are underway to replace LIBs with new electrochemical storage solutions, which are safe, stable, low cost and with higher energy density to power long-range electric vehicles and long-lasting portable electronics.
Replacing the traditional graphite-based anodes with Li metal, a “holy” anode with a high theoretical capacity of 3860 mAh/g, shows it a promising approach. At present, Li metal anode suffers from poor cycling efficiency and infinite volume change, raising operational safety concerns. Effective efforts include functional electrolyte additive, artificial solid-electrolyte interface and using host scaffolds to buffer the volume expansion have been taken to tackle its disadvantages. Among these, the method of using scaffolds continues to see rapid development.
Graphite, a classic Li anode, shows a great promising as an effective host scaffold, which possesses a low density and high electron conductivity. However, it is generally accepted that Li metal wets graphite poorly, causing its spreading and infiltration difficult. Previous methods to transforming graphite from lithiophobicity to lithiophilicity include surface coating with Si, Ag or metal oxide (lithiophobic indicates a large contact angle, while lithiophilic indicates a low contact angle between molten lithium and solid surface). However, such a change in liquid spreading behavior is due to the replacement of graphite by reactive coating. Consequently, it might be asked whether graphite is intrinsically lithiophobic or lithiophilic.
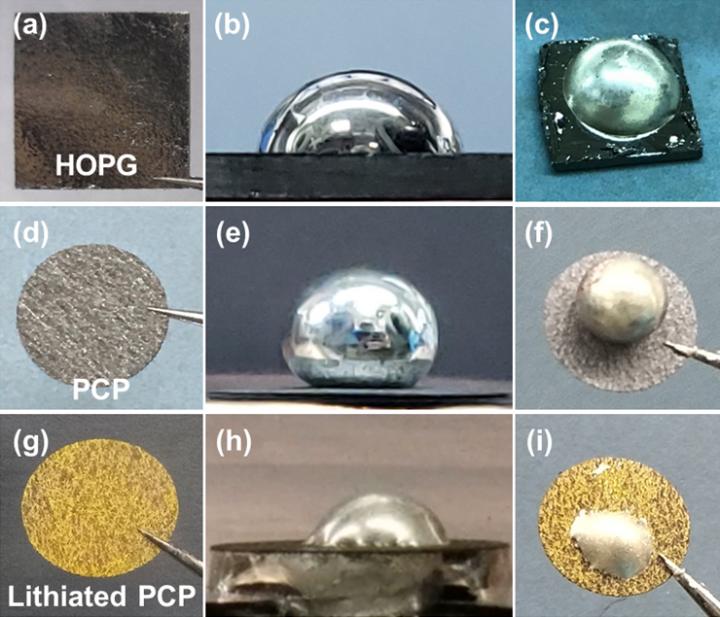
Herein, the wetting behavior of molten Li on different kinds of graphite-based carbon materials were systematically studied. Firstly, the highly oriented pyrolytic graphite (HOPG) was used as the test sample (Figure a). It is observed that HOPG substrate immediately allows an contact angle (CA) of 73° with Li metal (Figure b, c). To check this experiment against theory, ab initio molecular dynamics simulation was performed with a molten Li droplet (54 Li atoms)/graphite (432 C atoms, two-layered graphene) setup to prove that a clean (002) surface of graphite is intrinsically lithiophilic at 500K and the results also confirmed that lithium and graphite have good affinity.
However, the CA of Li metal on porous carbon paper (PCP, Figure d) is as high as 142°, which indicates PCP is lithiophobic (Figure e and f). This result which contradicted with previous conclusion that graphite is intrinsically lithiophilic prompted researchers to gain further understanding of the effect of surface chemistry to the wetting performance of Li metal and graphite. Compared with HOPG, it is found that PCP surface has a large number of oxygen-containing functional groups. These surface impurities will play a key role in pinning the contact line between Li metal and PCP, resulting in a lager apparent contact angle.
In order to demonstrate this assumption, the PCP was first lithiated by decreasing its electrochemical potential with molten Li metal (Figure g). During this process, the surface impurities of PCP will be eliminated as well. The following experiment shows that lithiated PCP exhibited a small CA of ~52°, which indicated a successful transition from lithiophobicity to lithiophilicity. Due to its porous structure of lithiated PCP, the Li metal rapid diffused through (Figure h and i). The DFT simulation revealed that lithiated graphite and graphite possessed similar wetting performance, demonstrating the elimination of the surface impurities would be the key reason for this transition of wetting performance from PCP to lithiated PCP. The graphite powder is further used to test its wettability with Li metal. After continue mixing, the graphite powder could be uniformly dispersed in the Li metal matrix, further confirming a lithiophilic property of graphite. Taking advantage of this discovery, a novel Li metal-graphite compositing method was proposed and Li-graphite composite anode with large area can be produced in a large scale.
This work not only systematically studies the wettability of Li metal and graphite-based carbon materials, but also provides a novel idea for the construction of Li-carbon composite anode materials, which is helpful for the development of high-energy Li metal batteries.
###
See the article:
Jian Duan, Yuheng Zheng, Wei Luo, Wangyan Wu, Tengrui Wang, Yong Xie, Sa Li, Ju Li, and Yunhui Huang,
Is graphite lithiophobic or lithiophilic?
Natl. Sci. Rev.
https:/
Media Contact
Yunhui Huang
[email protected]
Related Journal Article
http://dx.




