XSEDE-allocated Stampede2 supercomputer powers path-sampling molecular simulations
Credit: Malmir et al.
For a long time, nothing. Then all of a sudden, something. Wonderful things in nature can burst on the scene after long periods of dullness — rare events such as protein folding, chemical reactions, or even the seeding of clouds. Path sampling techniques are computer algorithms that deal with the dullness in data by focusing on the part of the process in which the transition occurs.
Scientists are using XSEDE-allocated supercomputers to help understand the relatively rare event of salts in water passing through atomically-thin, nanoporous membranes. From a practical perspective, the rate of ion transport through a membrane needs to be minimized. In order to achieve this goal, however, it is necessary to obtain a statistically representative picture of individual transport events to understand the factors that control its rate. This research could not only help make progress in desalination for fresh water; it has applications in decontaminating the environment, better pharmaceuticals, and more.
Advanced path sampling techniques and molecular dynamics (MD) simulations captured the kinetics of solute transport through nanoporous membranes, according to a study published online in the Cell journal Matter, January 2020.
“The goal was to calculate the mean first passage times for solutes irrespective of their magnitude,” said study co-author Amir Haji-Akbari, an assistant professor of chemical and environmental engineering at Yale University.
The team was awarded supercomputing time by XSEDE, the Extreme Science and Engineering Discovery Environment (XSEDE) funded by the National Science Foundation. The XSEDE-allocated Stampede2 system at TACC was used for the simulations in this study, in particular the Skylake nodes of Stampede2.
“XSEDE was extremely useful and indispensable to what we did,” Haji-Akbari said. “That’s because the underlying trajectories that are part of the forward flux sampling method are fairly expensive atomistic simulations. We definitively couldn’t have finished these studies using the resources that we have locally at the Yale lab.”
MD simulations were used to calculate forces in the system studied at the atomic level. The problem with MD is that even today’s most powerful supercomputers can only handle number crunching at timescales of a few hundred microseconds. The semi-permeable membranes under study that rejected certain solutes or ions had mean first passage times that could be much longer than the times accessible to MD.
“We used a technique called forward flux sampling, which can be equally used with equilibrium and non-equilibrium MD. The non-equilibrium aspect is particularly important for us because, when you’re thinking about driven solute or ion transport, you’re dealing with a non-equilibrium process that is either pressure-driven or is driven through external electric fields,” Haji-Akbari said.
One can get an idea for this by imagining salty water being pushed by pistons against a membrane skin that only squeezes water out, leaving the sodium and chloride ions behind.
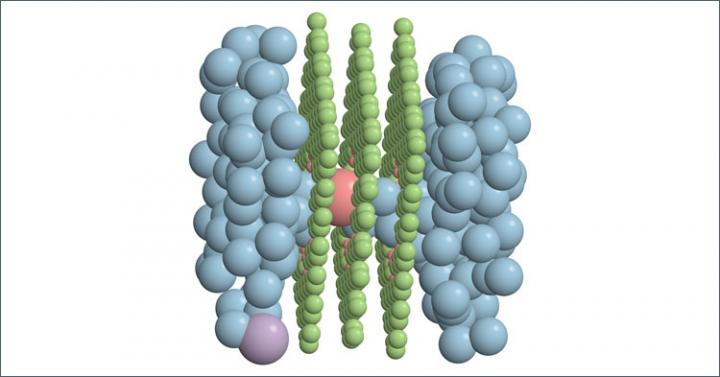
Haji-Akbari and colleagues used this experimental set-up with a special membrane with a nanopore through three layers of graphene. Surprisingly, even at that small scale, solutes that are supposed to be rejected can still fit.
“Geometrically, these solutes can enter the pores and pass the membrane accordingly,” Haji-Akbari said. “However, what seems to be keeping them back from doing that is the fact that, when you have a solute that is in water, for example, there usually is a strong association between that solute and what we call its solvation shell, or in the case of aqueous solutions, the hydration shell.”
In this example, solvent molecules can clump together, binding to the central solute. In order for the solute to enter the membrane, it has to lose some of these chunky molecules, and losing the molecules costs energy, which amounts to a barrier for their entrance into the membrane. However, it turns out that this picture, although accurate, is not complete.
“When you have an ion that passes through a nanoporous membrane, there is another factor that pulls it back and prevents it from entering and traversing the pore,” Haji-Akbari said. “We were able to identify a very interesting, previously unknown mechanism for ion transport through nanopores. That mechanistic aspect is what we call induced charge anisotropy.”
To give you a simple perspective of what that is, imagine a chloride ion that enters a nanopore. Once it approaches and then enters the nanopore, it sorts the remaining ions that are in in the feed. Because of the presence of that chloride inside the pore, it will be more likely for sodium ions in the feed to be closer to the pore mouth than the chloride ions.
“That is the additional factor that pulls back the leading ion,” Haji-Akbari explained. “You basically have two factors, partial dehydration, which was previously known; but also this induced charge anisotropy that as far as we know is the first time this has been identified.”
The science team based their computational method on forward flux sampling, which is parallelizable because the computational components do not interact that strongly with one another. “High performance computing is very suitable for using these types of methods,” Haji-Akbari said. “We have previously used it to study crystal nucleation. This is the first time that we’re using it to study ion transport through membranes.”
As supercomputers get better and better, they offer scientists tools to explore the unexplained in a more realistic way.
“We know that in real systems, the electronic cloud of any molecule or ion will be affected by its environment,” Haji-Akbari said. “Those kinds of effects are usually accounted for in polarizable force fields, which are more accurate, but more expensive to simulate. Because the calculation that we conducted was already very expensive, we didn’t afford to use those polarizable force fields. That’s something that we would like to do at some point, especially if we have the resources to do so.”
“Supercomputers are extremely useful in addressing questions that we can’t address with regular computing resources. For example, we couldn’t have done this calculation without a supercomputer. They’re extremely valuable in accessing scales that are not accessible to either experiments, because of their lack of resolution; or simulations, because you need a large number of computer nodes and processors to be able to address that,” Haji-Akbari concluded.
###
The study, “Induced Charge Anisotropy: A Hidden Variable Affecting Ion Transport through Membranes,” was published online in the Cell journal Matter in January 2020 (doi:10.1016/j.matt.2019.12.022). The study co-authors are Hessam Malmir of NASA Ames Research Center; Razi Epsztein of the Israel Institute of Technology; Menachem Elimelech and Amir Haji-Akbari of Yale University. The National Science Foundation funded the study.
Media Contact
Jorge Salazar
[email protected]
512-471-3980
Original Source
https:/
Related Journal Article
http://dx.




