László Kürti and team develop one-step process to make crucial precursor
Credit: Kürti Research Group/Rice University
HOUSTON – (Feb. 24, 2020) – In one pot, at room temperature, chemists at Rice University are able to make valuable pharmaceutical precursors they say could change the industry.
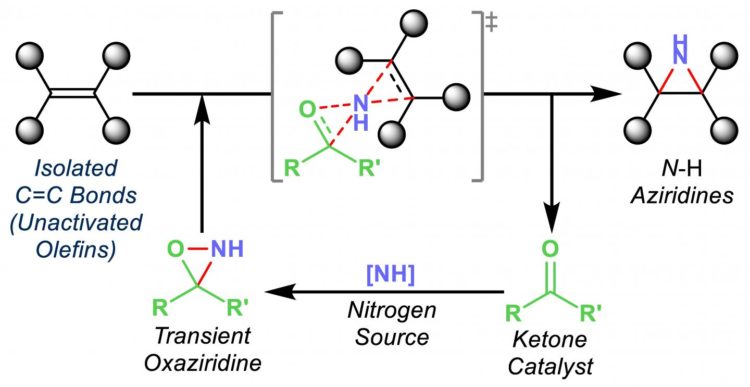
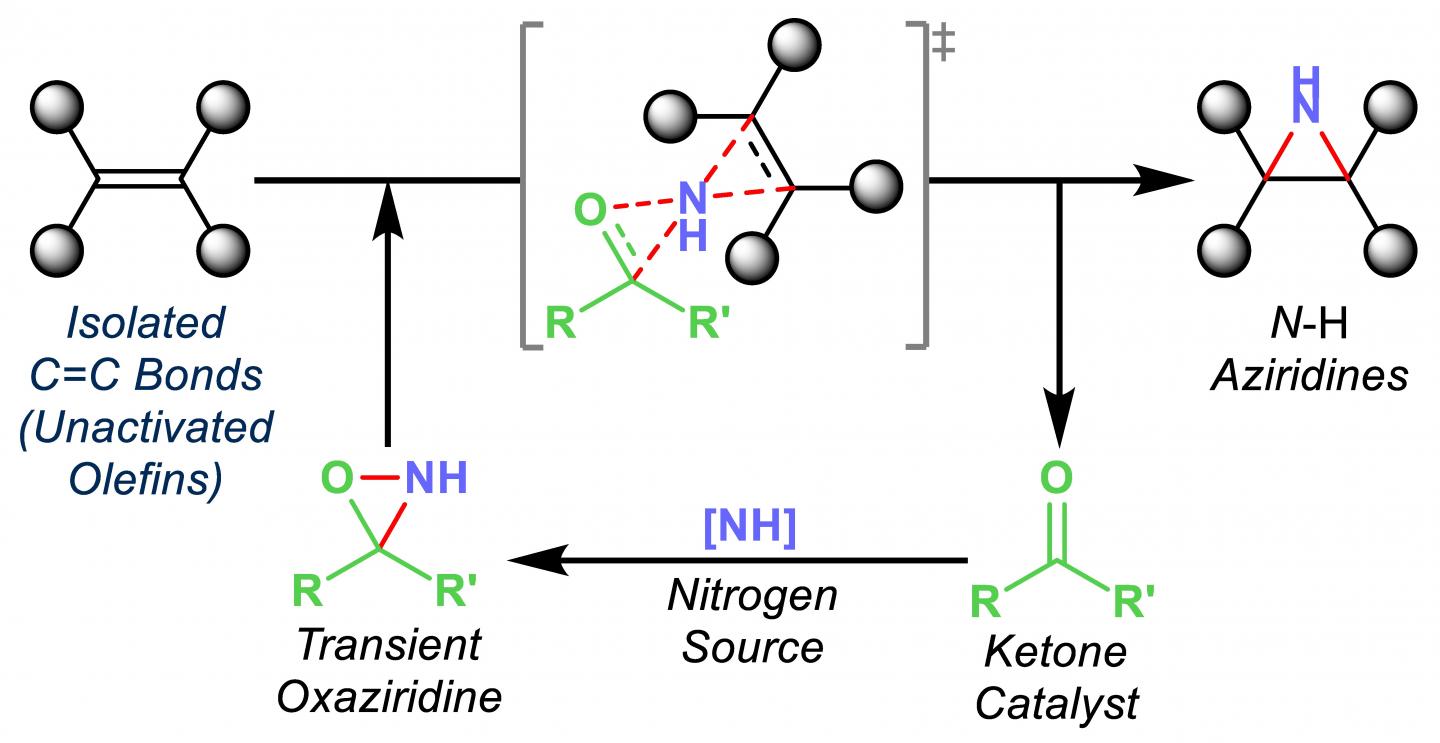
The Rice group of chemist László Kürti introduced an inexpensive organic synthesis technique that catalyzes the transfer of nitrogen atoms to olefins, unsaturated organic compounds also known as alkenes.
Exposed nitrogen atoms are critical to drug discovery. The Rice process combines nitrogen and hydrogen atoms in triangular aziridine products that are readily available to react with other agents.
Most important, Kürti said, is that his lab’s organocatalytic aziridination process transfers nitrogen to olefins that haven’t already been modified, or functionalized.
“These unactivated olefins are commodity chemicals, but very difficult to functionalize,” he said. “We are able to do that now with this chemistry under operationally simple and mild conditions.”
Turning them into nitrogen-containing small molecules makes them far more useful, he said. “You can then convert them to more complex molecules,” he said. “These N-H aziridines are essential building blocks.”
The lab detailed its new aziridination technique in Nature Catalysis.
Kürti and his crew have been stepping toward this point for years, first eliminating expensive catalysts from the process of transferring nitrogen to arylmetals, and later taking enol ethers and transferring nitrogen to them to make amino ketones, a feedstock for the chemical industry.
“The direct amination of enol ethers was a nice breakthrough because we didn’t need any catalyst,” he said. “The solvent was promoting the actual nitrogen-transfer process. Then we asked if we could replace the currently used precious metal catalysts with a small organic molecule at just a fraction of the cost to make aziridines.”
The new study provides a definitive yes. “This has been a dream of ours for a long time,” Kürti said.
Kürti and postdoctoral associate and co-author Zhe Zhou estimated the commercially available organic small molecule catalyst needed for the process is about 4,000 times less expensive than the rhodium-based catalysts in common use. They also make the process more sustainable.
“Everybody thinks catalysis is the answer for our problems, and in many cases it’s true,” Kürti said. “In a difficult reaction, a small amount of catalyst will accelerate the process and save time and money.
“But many people forget the cost of the catalyst, and whether it’s sustainable,” he said. “Unfortunately, it’s become pretty clear that we’re using high-value catalysts that contain precious metals. The world supply is limited, and the prices of these metals are at best erratic.”
The Rice process comes with one disadvantage, however. “It’s slower than the rhodium-catalyzed process,” Kürti said. “What we disclose here takes about six hours at room temperature, where the rhodium-catalyzed process, depending on the substrate, ranges between 10 minutes and a half hour.
“You definitely give up a little bit there,” he said. “But six hours is tolerable if you’re making big batches. That’s what I hope people will recognize in the long run.”
Kürti hopes to refine the process to control how the nitrogen attaches to the olefin and then, in turn, control the essential chirality, or handedness, of the product. The chirality of a drug is critical to how well it works, if at all.
Until then, the current process could be of great interest to industry, he said.
“Easier access to previously difficult-to-obtain precursors can actually influence the compound structures that chemists will make in the in the lab,” Kürti said. “Simple procedures that are straightforward to use tend to dominate in pharmaceutical drug development.”
###
Former Rice postdoctoral researcher Qing-Qing Cheng, now a postdoctoral researcher at the Scripps Research Institute, is lead author of the paper. Co-authors include associate professor Xinhao Zhang and graduate student Heming Jiang of the Peking University Shenzhen Graduate School and Shenzhen Bay Laboratory; Rice lecturer Juha Siitonen; and Daniel Ess, an associate professor of chemistry and biochemistry at Brigham Young University. Kürti is an associate professor of chemistry at Rice.
The National Institutes of Health, the National Science Foundation, the Robert A. Welch Foundation, Shenzhen STIC and the Shenzhen San-Ming Project supported the research.
Read the abstract at https:/
This news release can be found online https:/
Follow Rice News and Media Relations via Twitter @RiceUNews
Related materials:
Rice chemists show its hip to be square: http://news.
Kürti Research Group: http://kurtilabs.
Rice Department of Chemistry: https:/
Wiess School of Natural Sciences: https:/
Image for download:
https:/
A Rice University method to produce aziridines, building blocks in drug design, makes the process far less expensive and more environmentally friendly than current methods that use metal catalysts. (Credit: Kürti Research Group/Rice University)
https:/
Rice University researchers have further simplified their process to make essential precursor molecules for drug discovery and manufacture. From left: Zhe Zhou, Juha Siitonen, Qing-Qing Cheng (on screen) and László Kürti. (Credit: Kürti Research Group/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Jeff Falk
713-348-6775
[email protected]
Jade Boyd
713-348-6778
[email protected]
Media Contact
Jeff Falk
[email protected]
713-348-6775
Original Source
https:/
Related Journal Article
http://dx.